A new method to diffusion
bond superalloys
European and USA Patents pending
A.A. Shirzadi and E.R. Wallach
Department of Materials Science and Metallurgy
University of Cambridge
Pembroke Street, Cambridge CB2 3QZ, UK
Background
Nickel and
cobalt-base superalloys are especially suitable for the manufacture of
components to be used in high temperature applications, e.g. turbine blades and
rotary disks used in gas power generation plants and aircraft engines. Due to the extremely high cost of
manufacturing, maintaining and repairing such components, the joining of
superalloys has been of a major interest to the power plant and aerospace
industries, and a considerable amount of research is carried out in this
field. Despite recent developments
in the fusion welding of superalloys using laser or TIG welding processes, the
formation of hot cracks remains a major problem. Other joining methods, such as brazing and transient liquid
phase (TLP) diffusion bonding, normally require long bonding times and/or
post-bond heat treatments.
Therefore, none of the existing methods for joining superalloys has
proved entirely satisfactory to and viable for design engineers. Hence, further improvements of the
existing joining methods, as well as the development of new joining approaches,
are necessary in order to meet some of the more demanding requirements when
joining high performance materials.
A new diffusion bonding method
Diffusion
bonding is a process by which faying surfaces are brought into sufficiently
close contact using an applied pressure at elevated temperature to allow bond
formation by atomic interdiffusion across the joint interface. There are several hypotheses which have
been proposed to suggest how a bond is formed in a diffusion bonding process.[ ] One of these hypotheses emphasises the
effect of surface oxide layers on the joining process. It was proposed that the observed
differences in the weldability of various metals can be attributed to the
different properties of their surface films, and hence it is assumed that all
metals will bond if thoroughly cleaned surfaces are brought together within the
range of interatomic forces.
Although
surface oxide films can easily be removed by grinding the faying surfaces of an
alloy, a new oxide layer forms immediately due to the exposure of the ground
metallic surface to ambient oxygen.
The reformation of the surface oxide is virtually instantaneous for many
metallic alloys including most superalloys since they contain elements with a
high affinity for oxygen, e.g. Ni, Cr, Al, Co, Ti, W. Therefore, it would be beneficial to develop a method that
can remove the surface oxide and then prevent its reformation on the cleaned
faying surfaces prior to bonding.
A
new method for diffusion bonding nickel and cobalt-base superalloys has been
developed in this work, and is based on non-chemical oxide removal prior to the
bonding process. Using this
method, most of the stable oxides on the faying surfaces of the superalloy are
replaced with a very thin metallic layer.
The treated faying surfaces are believed to either be virtually
oxide-free or contain far less stable and less detrimental surface oxides than
the original surface oxide on an untreated surface. The details of this new
method are to be published when patent protection has been completed. However, it must be emphasised that the new oxide removal method is very rapid and also
neither requires the use of any sophisticated equipment nor is a costly
process.
Experimental procedure
Several
nickel-base superalloys, including directionally solidified DSR142, single
crystal SRR99, and a cobalt-base superalloy PWA647, were used in this
work. The compositions of the
alloys bonded are shown in Table 1.
|
Superalloy |
Cr |
Co |
Mo |
W |
Al |
Ti |
B |
C |
Si |
Zr |
Others |
|
|
|||||||||||
Inconel
718
|
18.3 |
0.1 |
2.85 |
|
0.5 |
0.92 |
0.003 |
0.02 |
0.08 |
0.01 |
0.08Mn, 0.0004S |
|
Inconel 738 |
16 |
8.5 |
1.75 |
2.6 |
3.4 |
3.4 |
0.01 |
0.17 |
|
0.1 |
1.75Ta, 0.9Nb |
|
C1023 |
15 |
10 |
8.5 |
|
4.2 |
3.6 |
0.006 |
|
|
|
|
|
DSR142 |
8.3 |
13 |
1.5 |
6 |
3.4 |
|
|
|
|
|
7Ta, 1.8Hf |
|
PWA647 |
23 |
Bal. |
0 |
|
0 |
0.2 |
0 |
0.6 |
0 |
0.5 |
10Ni, 7W, 3.5 Ta |
Table 1: Chemical compositions (wt%) of alloys used in this work (balance Ni except for cobalt-base superalloy PWA647.
Diffusion
bonding of these various superalloys was carried out after treating the faying
surfaces using the new oxide removal method. The samples were bonded in vacuum (10-4 mbar) at
a temperature between 900 to 1250ûC and under a pressure which ranged between 3
to 10 MPa. The precise parameters
were dependent on the particular alloy being joined. The bonding time for all samples was 1 hour. Some of the samples were post-bond
heat-treated at 1150ûC for 24 hours.
A few joints between dissimilar alloys were also produced to assess the
capability of the new method for producing more complex or multilayer
components.
Examination of bond line microstructures
Optical
microscopy, scanning electron microscopy (SEM) and X-ray energy dispersive
spectroscopy (EDS) were used to study the microstructures and compositions of
the bond lines. Figure 1 shows the
microstructures of the bonds made in two superalloys (C1023 and PWA647) with
equiaxed grains in the as-bonded condition. The microstructures of the bond lines are very similar to
those of the corresponding bulk alloys, and hence locating the bond lines
proved quite difficult. Grains
generally crossed the bond line and so left only a very small indication of the
initial location of the joint interface.
Having
heat-treated the already bonded C1023 sample at 1150ûC for 24 hours,
back-scatter electron SEM imaging and EDS were used to investigate any
compositional variations in the bond zone compared with that of the bulk. Figure 2 shows SEM micrographs of this
sample together with the results of EDS analysis on and away from the bond
line. It is clear that there is
little compositional variation, and any possible difference between the
compositions of the bond line and the bulk was below the detection limit of the
EDS analyser used (<0.1 wt%).
In
contrast, the joint interfaces in the bonded directionally-solidified
superalloy DSR142 and the single crystal SRR99 could easily be located due to
differences in grain alignment or phase distribution in the pieces bonded Ð see
Figure 3.
Further
examination, using SEM and EDS, of the bond in DSR142 revealed that a
discontinuous phase was formed on the bond line. There was a high concentration of heavy elements such as Ta
and Hf within this discontinuous phase Ð see Figure 4. However, despite the presence of this
phase, the bond strength still was excellent, probably due to the discontinuity
of these phases. The effect of a
post-bond heat treatment on the amount and distribution of this phase will be
investigated in future work.
A
dissimilar joint between the nickel-base superalloy Inconel 718 and the
cobalt-base superalloy PWA647 was also produced. Finger 5 shows the microstructure of the bond line and it is
clear that a substantial amount of interdiffusion across the joint interface
has occurred during the one hour bonding time. No continuous interfacial phase was observed at the bond
line and further investigation using SEM is in progress.
Evaluation of bond strength
In order to
evaluate the bond strengths of the samples, thin slices with thicknesses
between 300 mm
and 1 mm were cut and bent across the bond line. Although quantitative mechanical testing was not carried out
in this work, the results after severe bending are extremely promising. Most of the sliced samples showed no
preferential failure or even lack of ductility at the joint interface. In fact, the slices produced from
bonded samples behaved like monolithic pieces of the alloy tested.
Despite the
presence of a distinguishable bond line in the nickel-base DS alloy and single
crystal, their bond strengths and ductility were also comparable to those of
the parent alloys. Similarly, the
dissimilar joint between the nickel and cobalt-base superalloys, Inconel 718
and PWA647 respectively, had excellent bond strength. Figure 6 shows some bonded samples, including dissimilar
joints, which withstood severe mechanical deformation without showing any
failure of or preferential fracture on the bond line.
Conclusion
A new
diffusion bonding method, based on removing the surface oxide prior to bonding,
has been developed. Using this new
approach, diffusion bonds in nickel and cobalt-base superalloys were produced
with interfacial microstructures and compositions very similar to the bulk
alloys. The required bonding time
is about one hour which is substantially lower than those used in previous
diffusion bonding approaches, e.g. 10 to 48 hours. The results of severe mechanical tests
of the bonded samples, including DS, single crystal and dissimilar superalloys,
are very promising. The high
temperature properties of bonded samples currently are being investigated.

Figure 1. Optical micrographs of two superalloys bonded
using the new diffusion bonding method (etched). Brackets [ ] show approximate location of the bond line.
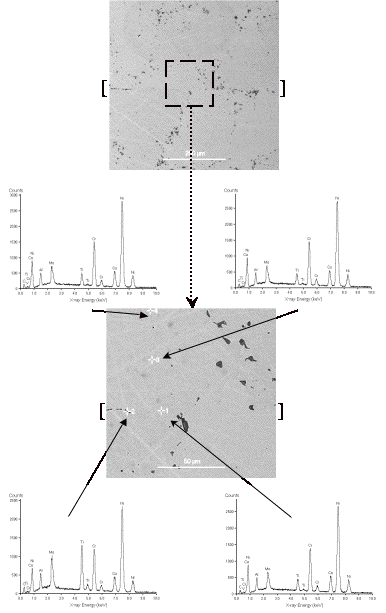
Figure 2. SEM micrograph of heat-treated bond in C1023 superalloy and the results of EDS analysis show the microstructure and composition of the bond line are very similar to the bulk alloy. Brackets [ ] show approximate location of the bond line.
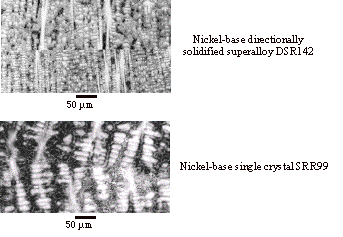
Figure 3. Optical
micrographs of a nickel-base directionally-solidified superalloy and a single
crystal bonded using the new diffusion bonding method (etched). The bond lines are clearly visible.
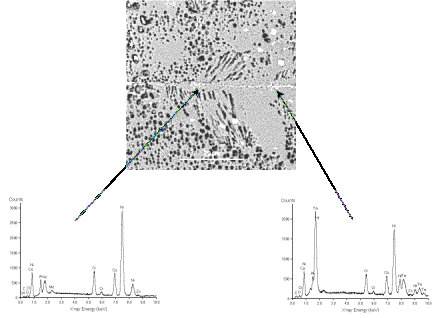
Figure 4. SEM micrograph of DSR142 superalloy and the results of EDS analysis show the formation of a discontinuous phase (bright) on the bond line containing heavy elements such as Ta and Hf.
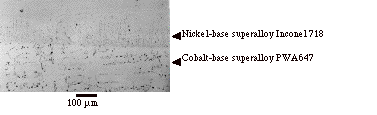
Figure 5. Optical micrograph of a dissimilar joint
between Inconel 718 and a cobalt-base superalloy PWA647 bonded using the new diffusion bonding method (etched).

Figure 6. Bonded samples of various nickel and cobalt-base
superalloys, including dissimilar and multilayer joints, which have been
subjected to severe mechanical deformation without showing any preferential
failure of the bondline.