
Precipitate Free Zones
H. K. D. H. Bhadeshia
It is often the case that precipitation does not occur uniformly throughout the microstructure during the heat treatment of a supersaturated phase. Regions in the proximity of a grain boundary are frequently found to be free of precipitates. These precipitate free zones (or PFZ's) occur for two reasons:
- The most common reason for the formation of PFZ's is that precipitates nucleate heterogeneously on vacancies. A grain boundary is a sink for vacancies so that regions adjacent to the boundary are unable to nucleate the precipitates, even though the matrix may be supersaturated with solute.
- The grain boundaries themselves are potent heterogeneous nucleation sites. Particles may nucleate first at these boundaries, thereby removing sufficient solute from the adjacent matrix. The solute-depleted region in the proximity of the boundary therefore remains precipitate-free.
More details can be found in Lecture 8.
Direct Evidence for Vacancy Depletion at Grain Boundaries
As stated above, excess vacancies tend to sink into grain boundaries, but if these boundaries are few and far between, then they can also precipitate as dislocation loops. The electron micrograph below shows a grain boundary and dislocation loops formed by the condensation of vacancies. There is a loop-free zone adjacent to the boundary, because the excess vacancies have sunk into the boundary instead of condensing into dislocation loops.
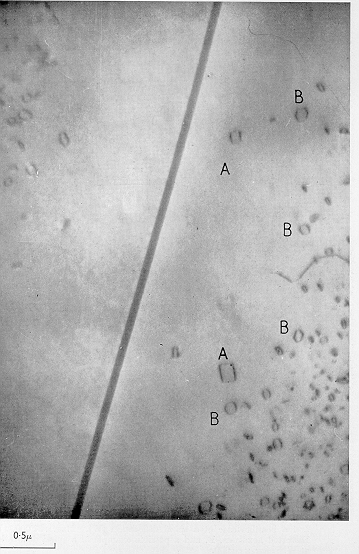
| Transmission electron micrograph, showing dislocation loops which have formed by the condensation of excess vacancies. The loops are not evident in the vicinity of the grain boundary, because there is a vacancy depleted zone at the boundary. Ignore the ledgends "A" and "B", which refer to different kinds of dislocation loops. The micrograph is reproduced with the permission of Taylor Francis Ltd., from Philosophical Magazine vol. 3 (1958) p.897, a paper written by Hirsch, Silcox, Smallman and Westmacott. |
Chromium-Niobium and Cr-Nb-Zr Alloys
Chromium forms Laves phases with the general formula Cr2X, where X=Ta, Hf, Nb, Zr or Ti. Laves phases generally have a high melting temperature, a relatively low density. They might therefore be exploited in the design of high-temperature structural materials.
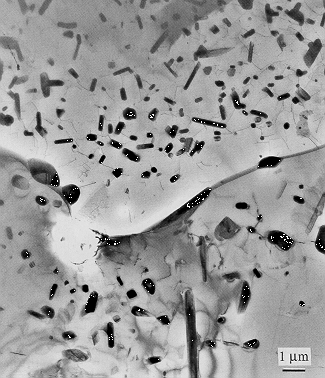
Cr-3Nb at% (Transmission Electron Micrograph).
|
Transmission electron micrograph, bright field image, of Cr-3Nb at.% alloy annealed at 1473 K for 100 h, showing precipitate-free zones at grain boundaries. The precipitates are Cr2Nb within a matrix which is Cr-rich. Reproduced with permission of Professor T. Takasugi, Osaka Prefecture University. Details can be found in Materials Science and Engineering A, 260A (1999) 108-123. |
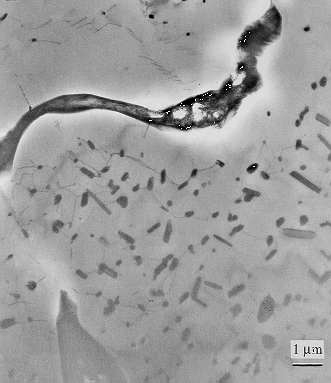
Cr-2Nb-1Zr at% (Transmission Electron Micrograph).
|
Transmission electron micrograph, bright field image, of Cr-2Nb-1Zr at.% alloy annealed at 1473 K for 100 h, showing precipitate-free zones at grain boundaries. The precipitates are Cr2(Nb,Zr) within a matrix which is Cr-rich. Notice intense precipitation at the grain boundary itself. Reproduced with permission of Professor T. Takasugi, Osaka Prefecture University. Details can be found in Materials Science and Engineering A, 260A (1999) 108-123. |
Aluminium Alloys
Aluminium alloys suffer a great deal from the formation of precipitate-free zones, partly because they are frequently strengthened using precipitation hardening, but also because they can contain high concentrations of vacancies.
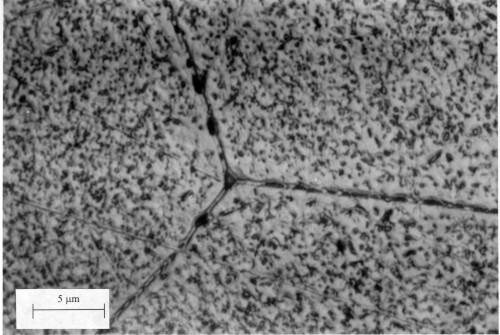
Al-4Cu wt% (high magnification, etched in NaOH)
|
The precipitate-free zones (PFZ) and grain boundary precipitates are visible in this image. The PFZ's are obviously regions of weakness. However, they have a more serious deterimental effect. The free energy of the PFZ is different from the remaining microstructure, so that corrosion currents arise in the presence of electrolytes (water). This leads to severe and unacceptable attack of the microstructure. This can be avoided by cladding the age-hardened sample with pure aluminium, a procedure adopted for the construction of airframes. |
Al-4Cu wt% (high magnification, etched in NaOH)

|
The precipitate-free zones (PFZ) and grain boundary precipitates. |
Remedies
PFZ's due to vacancy depletion can be avoided by heat treatment at a sufficiently low temperature (high driving force) where homogeneous nucleation becomes possible. However, this also means that the growth rate of the precipitates becomes painfully small, requiring very long heat treatment times. Thus, a sample where nucleation has been induced by a low temperature heat treatment can then be aged at a higher temperature where the growth rate is larger.
Even in this two-stage heat treatment, some growth must be promoted at the lower temperature. Otherwise the nuclei dissolve on raising the temperature if they become sub-critical in size, a process known as reversion.
Trace elements can be added which reduce the mobility of vacancies.
For further information see
- Light Alloys, by I. J. Polmear, 3rd edition, Edward Arnold, 1995.
- Phase Transformations in Metals and Alloys, by D. A. Porter and K. E. Easterling, 2nd edition, Chapman and Hall, 1992.
- Metals and Materials, by R. E. Smallman and R. J. Bishop, Butterworth-Heinemann, 1995.
- Aluminium Alloys: Structure and Properties, L. F. Mondolfo, Butterworths, 1976.







