Interface evolution and bond strength when
diffusion bonding materials with stable oxide films
A.A.
Shirzadi*, H. Assadi† and E.R. Wallach*
*Department of Materials Science and Metallurgy,
University of Cambridge, Pembroke Street, Cambridge CB2 3QZ, U.K.
†Department of Materials Science and Engineering,
Tarbiat Modarres University, PO Box 14115-143, Tehran, Iran
Abstract – The effects of stable surface oxides on the interface morphologies and strengths of aluminium diffusion bonds are reviewed. Previous approaches, proposed to overcome problems with surface oxides when joining aluminium alloys and composites, are described and compared for both solid-state diffusion bonding and conventional transient liquid phase (TLP) diffusion bonding. Non-conventional joining methods, particularly the new method of temperature-gradient TLP diffusion bonding and its capability of producing high-strength bonds reliably are also considered.
1. Introduction
Diffusion bonding is a process by which two nominally flat interfaces can be joined at an elevated temperature using an applied pressure for a time ranging from a few minutes to longer. The temperature is usually in the range of 0.5 – 0.8 Tm, where Tm is the absolute melting point of the material being joined. The interfacial pressure generally is sufficiently low to prevent large-scale deformation although localised deformation at the interface itself may be substantial. Two main variants of the process exist, solid-state and transient liquid phase (TLP). In the former, disruption of the oxide and surface films allows intimate metallic contact and hence bonding. In the latter, a liquid layer is formed during the bonding process and then, as a consequence of continued interdiffusion at the bonding temperature, isothermal solidification occurs to effect the bond.
The aim of diffusion bonding is to bring the surfaces of the two pieces being joined sufficiently close that interdiffusion can result in bond formation. However, there are two major obstacles that need to be overcome in order to achieve satisfactory diffusion bonds. Firstly, even highly polished surfaces come into contact only at their asperities and hence the ratio of contacting area to faying area is very low. Secondly in certain materials, the presence of oxide layers at the faying surfaces will affect the ease of diffusion bonding. For some metallic alloys, their oxide films either dissolve in the bulk of the metal or decompose at the bonding temperature (e.g. those of many steels, copper, titanium, tantalum, columbium and zirconium), and so metal-to-metal contact can be readily established at the interface. The joining of these materials is relatively straightforward and is not included in this review. However, if the oxide film is chemically stable, as for aluminium-based alloys, then achieving a metallic bond can be difficult. This review considers the diffusion bonding of this latter group of alloys.
In practice, because of inevitable surface roughness and also the presence of oxide layers on most faying surfaces, it is neither feasible to bring together the surfaces of two pieces within interatomic distances nor to establish complete metal-to-metal contact by simply putting two pieces together. In this paper, some aspects of the effects of surface oxides on interface morphology and bond strength are discussed, and a summary is provided of the existing approaches used to overcome the oxide problem when diffusion bonding, with the emphasis on aluminium-based alloys and composites.
2. Solid-state
diffusion bonding
There are several hypotheses1 to explain how a bond is formed in the solid-state diffusion bonding process, e.g. the “film hypothesis” which attributes the relative ease of joining various materials to differences in the properties of their surface films. In practice, regardless of which hypothesis or mechanism is considered, there is general agreement that all metals are assumed to bond if thoroughly cleaned surfaces are brought together within the range of interatomic forces. Hence the presence of surface oxides will inhibit bonding unless they can be removed or modified.
A considerable amount of work on modelling solid-state diffusion bonding has been carried out2, and generally there are two main aims. The first is to provide a basis for selecting appropriate process variables and the second is to obtain a detailed understanding of the mechanisms involved in order to further optimise the bonding process for different materials. However, in none of the existing analytical or numerical models has the effect of oxide film on the bond formation been considered2, 3. Hence, while some of the models can accurately predict the extent of bonding for metals with soluble oxides, they are of more limited use for alloys with stable oxides, such as aluminium alloys.
Previous experimental work on solid-state diffusion bonding of alloys with stable oxide layer has shown that the presence of the oxide film is the main barrier to successful bonding. Hence, the development of a method to disrupt the oxide layer would lead to significant improvements in bond integrity. Within the last forty years of investigation into the solid-state diffusion bonding of aluminium alloys, various approaches have been produced to overcome the oxide problem, and the major approaches are reviewed below.
2.1 Imposing substantial plastic deformation
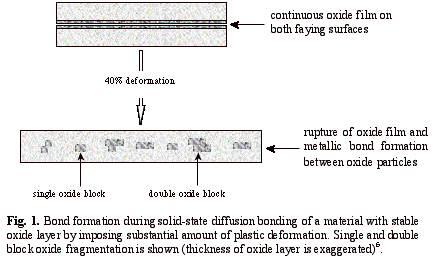 In
the solid-state diffusion bonding of aluminium alloys, the brittle and
continuous oxide layer can be broken up by imposing substantial plastic
deformation4, 5. The oxide film has a much lower ductility
(typically only a few percent) than the parent alloy and hence it ruptures when
parent alloys are subjected to a large amount of plastic deformation as it is
shown schematically in Fig. 1. Metal-to-metal contact is thus promoted as a
consequence of local disruption of the oxide film on both faying surfaces;
various ways by which this is achieved, including how the oxide fragments into
“single” and “double” blocks have been described6. Early work on
solid-state diffusion bonding of aluminium alloys in 19664, and more
recently in 19957, has shown that a minimum amount of deformation (~
40%) is required to produce bonds with reasonable strengths.
In
the solid-state diffusion bonding of aluminium alloys, the brittle and
continuous oxide layer can be broken up by imposing substantial plastic
deformation4, 5. The oxide film has a much lower ductility
(typically only a few percent) than the parent alloy and hence it ruptures when
parent alloys are subjected to a large amount of plastic deformation as it is
shown schematically in Fig. 1. Metal-to-metal contact is thus promoted as a
consequence of local disruption of the oxide film on both faying surfaces;
various ways by which this is achieved, including how the oxide fragments into
“single” and “double” blocks have been described6. Early work on
solid-state diffusion bonding of aluminium alloys in 19664, and more
recently in 19957, has shown that a minimum amount of deformation (~
40%) is required to produce bonds with reasonable strengths.
Although high strength bonds can be achieved by applying significant plastic deformation during the bonding process, this method has limited application due to the need for substantial deformation of the parent materials during the bonding process. Clearly, there are similarities and overlap between this approach and conventional pressure welding.
2.2 Enhancing microplastic deformation of
the surface asperities
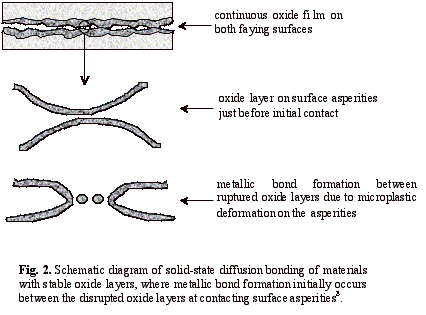
An alternative approach to overcoming the oxide problem in solid-state diffusion bonding is to use a fairly rough surface finish, which may lead to higher bond strengths than those obtained using polished surfaces. It is suggested8 that the local plastic deformation in the initial stage of the bonding process leads to rupture of the oxide film as the asperities deform and metallic contact is achieved - see Fig. 2. The rougher the surface, the greater is the plastic deformation of the asperities; therefore more oxide fracture will occur and consequently metal-to-metal bonding is improved. In early work9, based on electric resistance measurements of Al-Al bonds, it was suggested that faying surfaces treated by coarse emery papers produced many more metallic bonds than smooth faying surface. Subsequently the effect of surface roughness on the shear strength of Al-8090 bonds was studied10 and, despite considerable scatter in the results, it was concluded that higher bond strengths were achieved when a rougher surface preparation was adopted. These results are consistent with investigations into the influence of surface preparation on the bond strengths of Al-7475 bonds11. More recently, the effects of the size and shape of surface asperities on the interfacial contact process have been modelled12 and verified experimentally13
In contrast, low temperature solid-state bonding of copper, using different surface preparations, showed14 a significant reduction in the bond strength as the surface roughness increased. This seems to be inconsistent with the above hypothesis, namely that increased surface roughness improves the bonding behaviour of materials. This inconsistency is probably due to the different properties of the surface oxides of copper and aluminium; the soluble copper oxide layer is not as detrimental as insoluble aluminium oxide to the bonding process.
2.3 Presence of active alloying elements
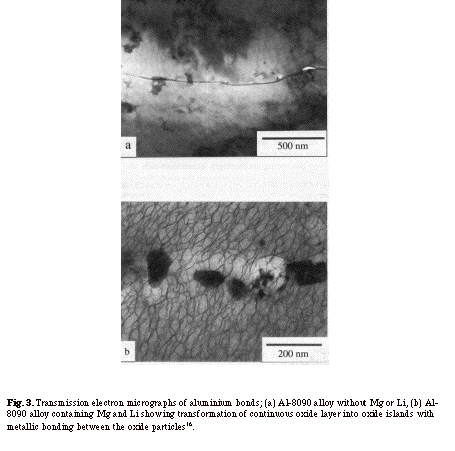
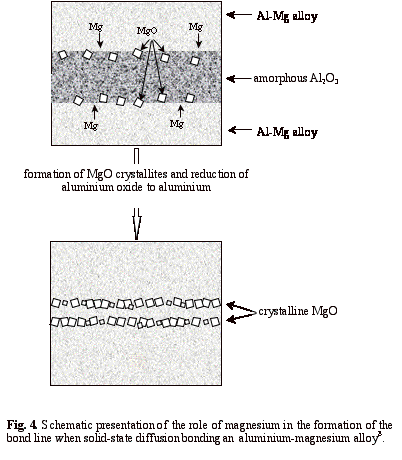
The use of active alloying elements, such as magnesium and lithium, when diffusion bonding aluminium-based alloys, has also been investigated8, 15, 16. According to this work, these active elements chemically interact with and break up the continuous and amorphous aluminium oxide layer at an interface to form an array of discrete particles. Figure 3a shows a TEM micrograph of the bond line of an aluminium 8090 alloy without magnesium or lithium where the oxide film remained continuous. In contrast, the bond line of aluminium 8090 alloy containing magnesium and lithium consisted of discrete oxide particles with metallic bonding between them - see Fig. 3b. The nature of the aluminium oxide at the interface also changed during the bonding cycle, from amorphous to crystalline8. A good correlation between bond strength and the extent of broken oxide was observed15, 16, leading to the conclusion that the greater the content of these elements, the greater the disruption of the oxide layer and consequently higher bond strengths are achieved. It was also concluded that magnesium is more effective than lithium in increasing bond strength. The effects of active elements in either shims or interlayers, placed at bond lines, have also been studied15. The use of Al-Mg interlayers improved the bond shear strengths by up to 50% in comparison with bonds made using pure aluminium interlayers. The above approach for the removal or disruption of stable oxide films, when solid state diffusion bonding, are shown schematically in Fig. 4.
2.4 Non-conventional solid-state bonding
methods
Diffusion bonding of an aluminium alloy at a temperature above the solidus line but below the liquidus line, e.g. at 580°C where solid and liquid phases coexist, aided the disruption of the oxide layers and promoted intimate contact at the interface17. This method was (surprisingly) referred to as solid-state diffusion bonding despite the formation of a liquid phase. A maximum shear strength of 270 MPa was achieved when the volume fraction of the liquid phase was 2~3% and this was increased to 400 MPa by a post-bond heat-treatment (no data on the parent metal properties were provided). However, when the volume of the liquid phase exceeded 3%, grain boundary cracking occurred and the bond line was associated with a large amount of porosity. No explanation was given for the fact that, despite the formation of the liquid phase at the grain boundaries, the base material could still withstand a bonding pressure of 1 MPa. Because of the high temperature used, the detrimental effects of melting on the microstructure and on the shape of the base material are expected to be substantial. In addition, any minor fluctuation during the bonding process would destroy the part being heated up to its solidus temperature. Hence, in practical terms, the process does not seem viable.
A different approach combined solid-state diffusion bonding and friction welding to join Al-6061 and Al-6061/SiC MMC18. In this method, a torsional force was exerted while an axial force was acting on the parts to be joined. One of the parts had a conical end in order to exude the worn oxide film from the interface. An important advantage of this method over diffusion bonding and friction welding was claimed to be the low bonding temperature (250-350°C) and short bonding time (~5 minutes), which reduced the plastic deformation during the bonding process. However, in contrast to diffusion bonding, the method is applicable only to parts with certain shapes (preferably with round cross-section) and which also have to be machined to provide conical ends. Also, unlike friction welding which is carried out in air, this method required a vacuum.
2.5 Summary on solid-state diffusion bonding of
aluminium alloys
Despite the extensive prior research on the solid-state diffusion bonding of aluminium alloys, this approach has failed to reliably produce high strength joints due to the presence of insoluble oxides at the interfaces of the parts being joined. The resulting wide scatter in bond strengths is a particularly discouraging feature of solid-state bonding, as are the poor fatigue properties. Non-conventional solid-state diffusion bonding processes require extreme bonding conditions, restricted joint configurations and/or complicated equipment in their attempts to improve reliability; therefore these approaches will tend to have limited application.
3. Transient liquid
phase (TLP) diffusion bonding
TLP diffusion bonding is a promising method for joining materials with stable oxide films as the presence of a liquid phase between two faying surfaces can accelerate the joining process by disrupting any oxide layers which remain stable at the bonding temperature. The formation of the liquid phase generally is achieved by inserting an interlayer with suitable composition or, but less popular, by increasing the bonding temperature above the solidus temperature of the interlayer. In the former case, the interlayer either has a lower melting point than the alloy being joined (e.g. zinc for aluminium alloys) or lowers the melting point locally as a consequence of the formation of a liquid eutectic phase arising from solid-state interdiffusion at the bonding temperature (e.g. copper for aluminium alloys). The liquid phase subsequently solidifies isothermally as a consequence of continued diffusion at the constant bonding temperature. This distinguishes the approach from brazing in which the interlayer solidifies only when the bond is cooled.
In a different approach, but combined with TLP diffusion bonding, attempts were made to remove the aluminium oxide using in-situ ion bean cleaning before copper sputtering in ultra-high vacuum19, 20. Using this technique, Al-8090 bonds with shear strengths up to 190 MPa were produced, approximately 90% of the shear strength (210 MPa) of the parent material. However, the complicated surface preparation method used for oxide removal is very restrictive and therefore is likely to have only laboratory application.
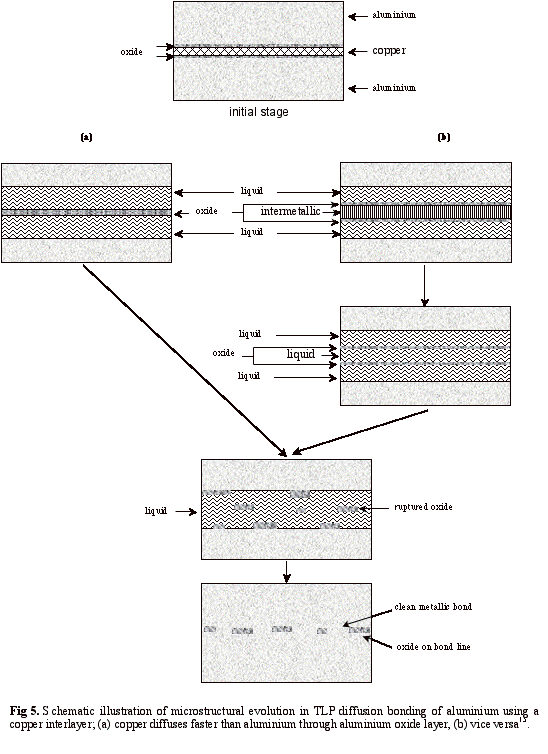
The effect of oxide layers on the formation of the liquid phase and the exact manner in which the microstructure develops is unclear. For instance in the case of bonding aluminium alloys using copper interlayers, the relative ease of diffusion of either copper or aluminium through the amorphous alumina surface layer, which is necessary to form the liquid eutectic phase at the bonding temperature, is unknown. In previous work15, the formation of a liquid phase for two cases in which copper or aluminium have fast diffusivity into the oxide layer was discussed. Consider the first case, and assuming copper diffuses through the oxide at a faster rate than aluminium, aluminium-copper eutectic will form as a liquid on both sides of the oxide films. Therefore the oxide will be surrounded by the liquid phase. The widths of the liquid layers on both sides of the oxide film increase until the copper interlayer is completely exhausted as it is shown in Fig. 5a. Although there has been no direct observation of the physical stability of the oxide layer surrounded by such liquid layers, it is reasonable to assume that the extremely thin and fragile oxide film will break up into small fragments during this process. As the liquid then solidifies isothermally due to continued interdiffusion, both advancing solid/liquid interfaces will push the fragments of oxide towards the final location of the interface where they agglomerate. The second, and less likely, case arises when aluminium diffuses through the oxide film faster than copper. This will result in the copper interlayer becoming increasingly aluminium-rich and the intermetallic phase, θ, forms between the oxide films. At the same time, the liquid eutectic phase will form between each oxide layer and its corresponding base aluminium due to the simultaneous diffusion of copper into the aluminium base metal. As the diffusion of aluminium continues, the change in composition leads to the formation of eutectic liquid from the intermetallic phase, forming a third liquid layer between the oxide films – see Fig 5b. Then, having formed these liquid phase regions, microstructural evolution will proceed as in the previous case where copper diffusion dominated.
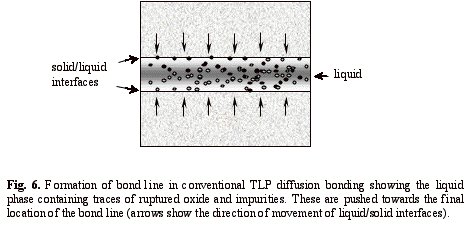
In summary, regardless of the bond formation mechanism, any insoluble oxide particles and impurities trapped within the liquid phase are pushed forward by the advancing solid-liquid boundaries during isothermal solidification in TLP diffusion bonding. Eventually, these particles agglomerate at the bond line and hence prevent metal-to-metal contact from being fully established. Figure 6 shows the mechanism of formation of a planar bond line containing impurities in conventional TLP diffusion bonding, and which is consistent with the TEM investigations carried out in earlier work15.
4. Summary of
solid-state and TLP diffusion bonding
It can be concluded that the bond line of materials with stable oxide films, whether diffusion bonded in the solid-state or using a TLP approach, will contain impurities and discontinuities which inhibit the formation of an ideal metallic bond. Also these bonds are normally associated, regardless of the type of bonding process, with planar interfaces6, 8, 15, 16, 19-24. Therefore, the shear strengths of such joints are expected to be somewhat less than the shear strengths of the corresponding parent materials, as has been observed experimentally. In addition, the random distribution of the oxide particles at these normally flat interfaces causes a wide scatter in the mechanical strengths of the bonds. Consequently, design engineers do not consider diffusion bonding a reliable method for joining these types of materials for commercial applications.
5.
Temperature-gradient (TLP) diffusion bonding
Imposing a temperature gradient and controlling the
temperature profile when TLP diffusion bonding can result in the formation of
non-planar interfaces3. This is a consequence of morphological
instability at the solid/liquid interface during solidification of the liquid
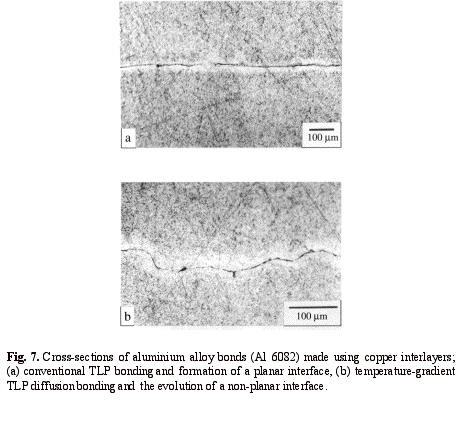
layer at the bonding temperature21, 25. Figure 7 shows
cross-sections of aluminium bonds made using conventional TLP diffusion bonding
and temperature gradient TLP diffusion bonding methods. Using this new method
of TLP diffusion bonding, reliable joints with shear strengths as high as those
of the parent material, even in aluminium-based alloys and composites, have
been produced3, 26. Two theoretical analyses of this new method have
been presented elsewhere27-29. These two complementary models allow
predictions of the bonding time, the interface morphology, and in general, the
bonding conditions which result in the formation of non-planar bond lines when
using this new bonding method28, 29.
The excellent bond strengths obtained when using this new method are possibly due to the higher metal-to-metal contact along the non-planar interfaces as compared to the planar interfaces associated with either conventional TLP or solid-state diffusion bonding processes. To verify this assumption, the fracture surfaces of Al-6082 bonds made using the two different methods of conventional TLP diffusion bonding and temperature-gradient TLP diffusion bonding were compared using scanning electron microscopy (SEM). Substantial differences in the topographies of the fracture surfaces due to the imposed temperature gradient are observed. The apparent fracture surface area increases dramatically due to the development of a sinusoidal interface during the bonding process. This effect can be clearly observed by comparing the SEM micrographs in Fig. 8, and is consistent with the bond cross-sections in Fig. 7.

Having optimised the bonding conditions and the interlayer thickness for the alloy Al-6082, bonds with shear strengths as high as that of the parent alloy were achieved25, 26. Figure 9 shows the cross-section of a sheared sample which withstood an applied shear stress of 251 MPa; the maximum and minimum shear strengths of the parent material subjected to the same thermal cycle were 237 and 252 MPa respectively.
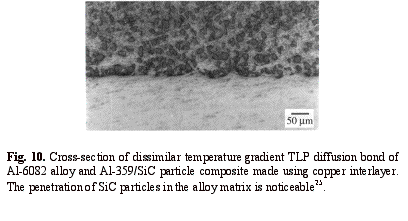
Using the new method, an Al-359/SiC composite was bonded to the Al-6082 alloy to produce a dissimilar joint. The bond line microstructure of a dissimilar bond made using the new method and a copper interlayer is shown in Fig. 10. The formation of a sinusoidal interface resulted in impingement of the SiC reinforcement into the alloy matrix, and this increased the shear strength of the joint up to 202 MPa3, 25. The shear strengths of the composite and the alloy, subjected to the same heat treatment, were 233 and 206 MPa respectively.

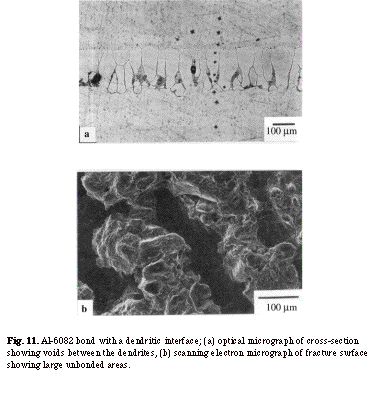
The wavy morphological instabilities at the interface can, nevertheless, have a detrimental effect on bond strength if they develop into fully dendritic microstructures3, 21. Figure 11 shows the cross-section and fracture surface of an Al-6082 bond with a fully dendritic interface. Due to the presence of voids and large unbonded areas between the dendrites, the bond strengths associated with this type of interface were very low. Thus the highest bond strengths are, in fact, achieved by controlling the level of waviness of the bond line. This means that, for a successful bonding process, it is important to promote the formation of interfacial instabilities, but it is equally important to prevent the excessive growth of such instabilities in order to avoid unfavourable microstructures. Hence, in order to obtain a bond line with a desired waviness (e.g. sinusoidal), it is essential to understand the factors influencing bond line morphology29.
6. Bond interface of aluminium metal matrix composites (MMCs) with
particle reinforcement
6.1 Solid-state diffusion bonding of aluminium MMCs
The bond line of an MMC-MMC joint generally consists of three different interfaces: matrix/matrix, matrix/particle and particle/particle. Obviously, the area fractions of these interfaces depend on the volume fraction and distribution of the reinforcement on each of the two faying surfaces. A proposed model shows that insertion of a matrix interlayer into a MMC-MMC bond interface may increase or reduce the area fraction of the matrix/particle interface30. According to this model, the presence of either particle/particle or matrix/particle interfaces leads to a lower shear strength of the bonded composite, compared to that of the bonded matrix alloy (i.e. with 100% matrix/matrix interface). This is explained as follows: firstly, almost no bond strength arises from a particle/particle interface and, secondly, the strength of a matrix/particle bond is assumed to be always lower than that of a matrix/matrix bond. The work30 assumes a flat interface when the two surfaces are brought together during the diffusion bonding process.
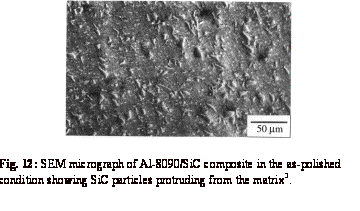 Examination
of a polished Al-8090/SiC composite revealed3 that the reinforcement
particles stick out of the polished surfaces - see Fig. 12. This is simply due
to the faster abrasion of the relatively soft aluminium matrix, compared with
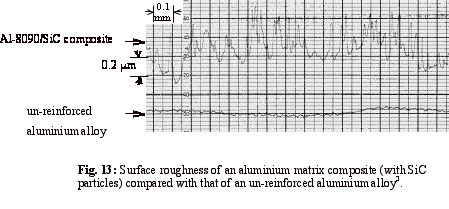
that of the hard SiC particles. Figure 13 shows the results of profilometry
carried out on the surfaces of an Al-8090/SiC composite and an un-reinforced
aluminium alloy, both polished in exactly the same way. As expected, due to the
protruding SiC particles, the surface roughness of the composite is much higher
than that of the alloy.
Examination
of a polished Al-8090/SiC composite revealed3 that the reinforcement
particles stick out of the polished surfaces - see Fig. 12. This is simply due
to the faster abrasion of the relatively soft aluminium matrix, compared with
that of the hard SiC particles. Figure 13 shows the results of profilometry
carried out on the surfaces of an Al-8090/SiC composite and an un-reinforced
aluminium alloy, both polished in exactly the same way. As expected, due to the
protruding SiC particles, the surface roughness of the composite is much higher
than that of the alloy.
 In
light of the above observations, it would be reasonable to assume that, during
diffusion bonding of the composite, some of the hard SiC particles on each
faying surface can easily penetrate into the much softer aluminium matrix on
the other surface. The depth of penetration depends on the shape of the
particle and the bonding conditions, and can reach up to 0.6 mm for
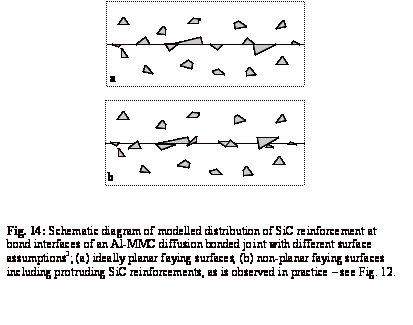
the composite used in this work. Figure 14a shows the distribution of particles
at the interface of an Al-MMC joint for an ideally planar interface. In
contrast, and consistent with the protrusion of the reinforcement, the bond
line of a joint made of non-planar faying surfaces is associated with interlocked
reinforcement particles along the bond interface - see Fig. 14b. The latter
would be expected to give higher bond strengths because of the interlocking
effect and also due to the local rupture of the oxide layer on each faying
surface by the protruding SiC particles from the other faying surface.
In
light of the above observations, it would be reasonable to assume that, during
diffusion bonding of the composite, some of the hard SiC particles on each
faying surface can easily penetrate into the much softer aluminium matrix on
the other surface. The depth of penetration depends on the shape of the
particle and the bonding conditions, and can reach up to 0.6 mm for
the composite used in this work. Figure 14a shows the distribution of particles
at the interface of an Al-MMC joint for an ideally planar interface. In
contrast, and consistent with the protrusion of the reinforcement, the bond
line of a joint made of non-planar faying surfaces is associated with interlocked
reinforcement particles along the bond interface - see Fig. 14b. The latter
would be expected to give higher bond strengths because of the interlocking
effect and also due to the local rupture of the oxide layer on each faying
surface by the protruding SiC particles from the other faying surface.
6.2 TLP diffusion bonding of aluminium MMCs
The bond line of a TLP diffusion-bonded composite is normally associated with agglomerated reinforcement particles. Reinforcement particles are displaced from their original locations as the liquid phase forms, and then are pushed ahead of the liquid-solid interface during solidification so that they end up located in the bond centre line - see Fig. 15. This segregation of particles depends on the amount of liquid phase (which is a function of the nature and thickness of the interlayer, and the bonding temperature) and the size of the particles. The effect of particle size (Al2O3) on the segregated layer in a dissimilar joint of Al-6061/Al2O3 and alumina has been studied31 and, not surprisingly, it was concluded that the width of the segregated layer increases as the particle size decreases. This is due to the fact that smaller particles are more easily pushed forward by the advancing liquid/solid interface, whereas larger particles are left behind the moving boundary.

In later work3, 32, experimental results showed that joints in Al-809/SiC composite with shear strengths as high as 221 MPa can be achieved. This value is greater than the upper bound value for the shear strength of a TLP diffusion-bonded Al-8090/SiC composite as predicted by the previous work30. Due to the presence of the liquid phase around the reinforcement particles in the bond region, the formation of particle/particle interfaces is doubtful since a liquid film would be expected to exist between contacting particles. Because of this and also due to the massive penetration of the particles at the bond line, generalising the above model for a TLP diffusion bond is even less realistic than for a solid-state diffusion bond.
6.3 Summary on diffusion bonding of aluminium MMCs
Despite substantial differences in the mechanisms responsible for particle distributions at bond lines when solid-state or TLP diffusion bonding MMCs, it is concluded that the reinforcement particles form non-planar interfaces compared to the bond lines in un-reinforced alloys. The assumption of a microscopically planar interface is not justifiable in either case. The formation of a non-planar interface can be of benefit in increasing the bond strength. The proposed model30 seems to be too simple to accurately predict bond strengths as it ignores the presence of a non-planar interface in composite joints due to the protruding particles and also the formation of liquid around the reinforcement particles when TLP diffusion bonding. A more comprehensive investigation is required to achieve a reasonable correlation between the area fractions of different interfaces and the bond strength of a composite joint. It also should be remembered that interactions may also occur between the reinforcement particles and the composite matrix or liquid phase during bonding. Such interactions, including the formation of intermetallic phases, are likely to affect bond strengths considerably. Such interactions for a Ti-based composite containing SiC reinforcement have been described33.
7. Conclusions
The presence of stable oxide films on the faying surfaces of some materials is one of the major barriers in achieving high strength and reliable diffusion bonds. Hence, aluminium-based alloys and composites are particularly difficult to diffusion bond. The surface oxides on such materials are physically very adherent, chemically stable and insoluble in the aluminium matrix at all temperatures, and so prevent full metal-to-metal contact from being established at the joint interface. During the last few decades, different approaches have been developed to circumvent the oxide problem when solid-state diffusion bonding. Most of these approaches are based either on physical disruption of the oxide film (by imposing plastic deformation during bonding), or chemical decomposition of the oxide using reactive elements (e.g. using Mg or Li as alloying elements within the base alloy or interlayer). Despite some progresses in improving bond integrity using these approaches, the solid-state diffusion bonding of aluminium alloys remains unsuccessful and interest in further research in this field seems to have declined substantially in the recent years. Non-conventional approaches, such as removing oxide layer in vacuum before the deposition of an interlayer or bonding at a temperature above solidus temperature, have limited application as they require extreme bonding conditions and/or complicated equipment.
Transient liquid phase (TLP) diffusion bonding is a promising method that has the potential to overcome the oxide problem. The formation of a liquid phase can result in fragmentation of the continuous oxide surface layers and therefore bond strengths can be improved substantially. However, following isothermal solidification, which is inherent in this process, fairly flat interfaces containing oxide particles and impurities form and such interfaces naturally have detrimental effects on the resulting bond strengths. Thus, regardless of the type of bonding process, whether solid-state or conventional TLP, the bond lines of materials with stable oxide films will contain impurities and discontinuities which inhibit the formation of ideal and complete metallic bonds.
Temperature gradient TLP diffusion bonding is a new approach capable of producing bond lines with various morphologies (e.g. sinusoidal to fully dendritic) by controlling the temperature profile across the liquid phase during bonding. Although oxide and impurities remain at the interfaces when using this new method, bond strengths and reliability increase dramatically due to the higher metal-to-metal contact along the non-planar interfaces as compared to the planar interfaces associated with either conventional TLP or solid-state diffusion bonding processes. Using this method, reliable bonds with shear strength as high as those of parent materials have been produced in aluminium alloys. It is likely that this new method will be of benefit when joining other materials with stable oxide films.
References
1. Kazakov N.F., Diffusion Bonding of Materials, Pergamon Press, 1985.
2. Wallach E.R., Trans. JWRI, 1988, 17 (1), 135.
3. Shirzadi A.A., PhD thesis, University of Cambridge, UK, 1998.
4. Cline C.L., Welding Research Supplement, Nov. 1966, 481s.
5. Hauser D., Kammer P.A. and Dedrick J.H., Welding Research Supplement, Jan. 1967, 11s.
6. Cantalejos N.A. and Cusminsky G., J. Inst. Met., 1972, 100, 20.
7. Urena A., Gomez de Salazar J. M. and Escalera M. D., Metal Matrix Composites; Key Engineering Materials, Trans. Tech. Publications Ltd., Switzerland, 1995, 104-107, 523.
8. Dray A.E., PhD. thesis, University of Cambridge, UK, 1985.
9. Enjyo T., Ikeuchi K. and Akikawa N., Trans. JWRI, 1978, 7 (2), 97.
10. Ricks R.A., Mahon G.J., Parson N.C., Heinrich T. and Winkler P.J., Proc. Conf. Diffusion Bonding 2, Cranfield Institute of Technology, UK, March 1990, ed. Stephenson D.J., Elsevier, Amsterdam, 69.
11. Tensi H.M. and Wittmann M., Proc. Conf. Diffusion Bonding 2, Cranfield Institute of Technology, UK, March 1990, ed. Stephenson D.J., Elsevier, Amsterdam, 101.
12. Takahashi Y. and Tanimoto M., Trans. ASME, 1995, 117, 330.
13. Takahashi Y. and Tanimoto M., Trans. ASME, 1995,117, 336.
14. Nicholas N.H., Nichting R.A., Edwards G.R. and Olson D.L., Proc. Conf. Recent Trends in Welding Science & Technology, 1990, ed. David S.A. and Vitek V.M., ASM International., 547.
15. Maddrell E.R., PhD thesis, University of Cambridge, UK, 1989.
16. Maddrell E.R., Ricks R.A. and Wallach E.R., Proc. Conf. Aluminium-Lithium 5, Williamsburg, Virginia, USA, March 1989, Materials and Component Engineering Publications Ltd., 451.
17. Enjo T. and Ikeuchi K., Trans. JWRI, 1984, 13 (2), 63.
18. Yokota T., Otsuka M., Haseyama T., Ueki T. and Tokisue H., Mater. Sci. Forum, 1997, 242, 225.
19. Dunford D.V., Partridge P.G. and Gilmore C.J., Proc. Conf. Diffusion Bonding 2, Cranfield Institute of Technology, UK, March 1990, ed. Stephenson D.J., Elsevier, Amsterdam, 130.
20. Gilmore C.J., Dunford D.V. and Partridge P.G., J. Mater. Sci., 1991, 26, 3119.
21. Shirzadi A.A. and Wallach E.R., Sci. Technol. Weld. Joining, 1997, 2 (3), 89.
22. Church S., Day J. and Wild B., Mater. World, 1996, 4 (7), 385.
23. Dunford D.V. and Partridge P.G., J. Mater. Sci., 1990, 25, 4957.
24. Bushby R.S. and Scott V.D., Mater. Sci. Technol., 1995, 11. 753.
25. Shirzadi A.A., Welding in the World, 1998, 41 (5), 435.
26. UK Patent 9709167.2, 6 May 1997 (International Patent pending).
27. Shirzadi A.A. and Wallach E.R., Proc. Conf. Solidification and Gravity 2000, Miskolc University, Hungary, April 1999, 2000 Trans. Tech. Publications Ltd., Switzerland, 351.
28. Shirzadi A.A. and Wallach E.R., Acta Mater., 1999, 47 (13), 3551.
29. Assadi H., Shirzadi A.A. and Wallach E.R., to be published in Acta Mater., 2001, 49 (1).
30. Partridge
P.G. and Dunford D.V., J. Mater. Sci.,
1991, 26, 2255.
31. Zhai Y. and
North T.H., J. Mater. Sci., 1997, 32,
5571.
32. Shirzadi A.A. and Wallach E.R., Mater. Sci. Technol., 1997, 13 (2), 135.
33. Kuriyama K. and Wallach E.R., Proc. Conf. Third International Conference on Brazing, High-Temperature Brazing and Diffusion Welding, Aachen, Germany, November 1992, Deutscher Verlag fur Schweisstechnik DVS-Verlag GmbH (Germany), 158.