Iridium
Iridium has a face-centred cubic crystal structure. It has a density of 22560 kg m-3, that is second only to osmium, and melts at 2446°C. According to Pugh*, materials with a large ratio of the shear modulus to bulk modulus will tend to be brittle at ambient temperature. In simple terms, this is because the shear modulus controls plasticity whereas the bulk modulus deals with cleavage. Hence, a large ratio makes plastic deformation, which is associated with ductility, more difficult. Iridium is a metal with a large modulus ratio and is indeed brittle even though it has a face-centred cubic crystal structure..
*Pugh SF. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. The London, Edinburgh, and Dublin Philosophical Magazine and Journal of Science. 1954 Aug 1;45(367):823-43.
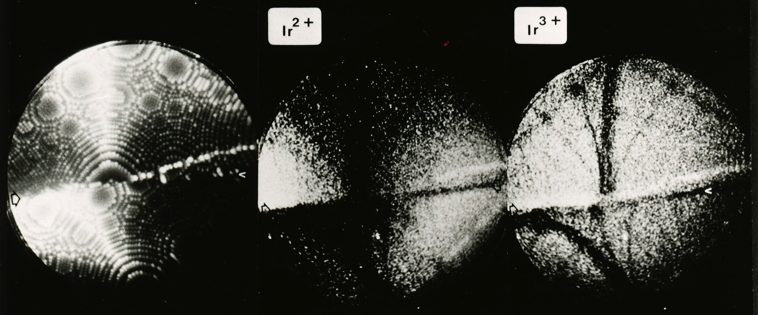
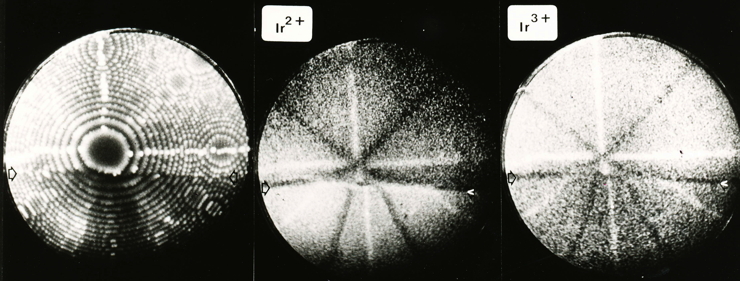
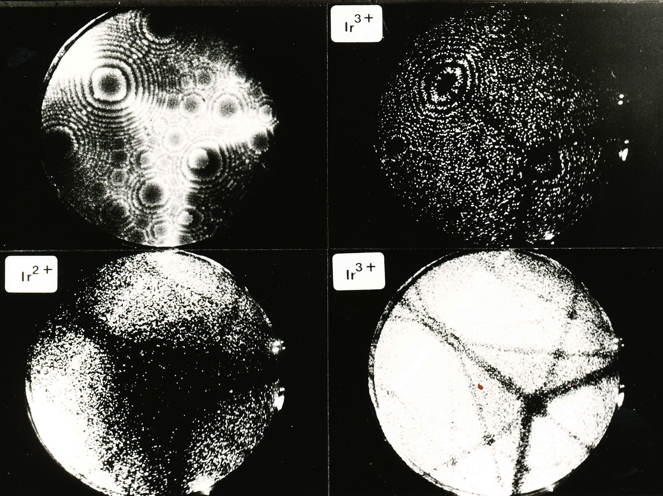
Iridium contributed a lot to the early development of the field ion microscope when the interpretation of images was not clear. Iridium and other platinum group metals have a large field evapration limit, and hence are stable to large electrical fields at cryogenic temperatures. In field ion microscopy, the tip of an electrically charged sharp needle specimen (radius about 100 nm) is in effect radially projected on to a fluorescent screen to produce enormous magnification, permitting the location of atoms to be imaged.
The imaging can involve an imaging gas (such as He or Ne), where the gas atoms are ionised at the atoms on the tip, and hence follow the field trajectories to magnify the atom locations. Alternatively, supplemental electrical pulses can induce the atoms to leave the tip in ionised form to follow the field trajectories and reach the imaging screen.
The following photographs are from work by A. R. Waugh, E. D. Boyes and M. J. Southon of the University of Cambridge. See, for example, Surface Science 61 (1976) 109-142. They have been provided courtesy of Dr Sally Waugh.
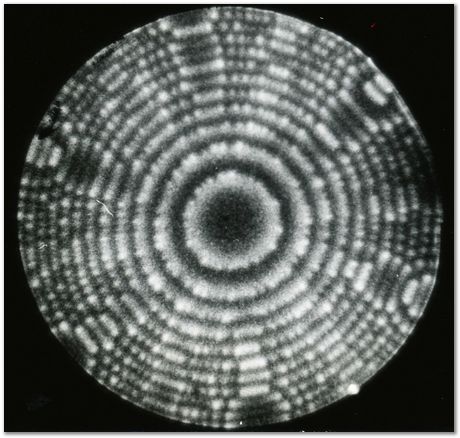
Helium field ion image of an iridium specimen, 78 K. |

Field desorption image. |
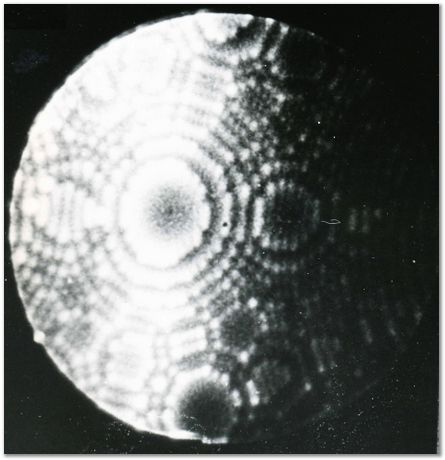
Helium FIM image of the {113} planes of iridium, 78 K. |
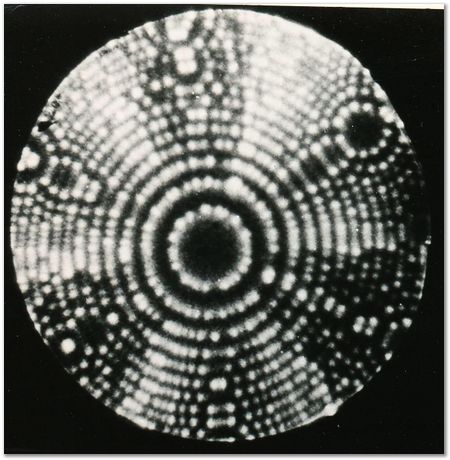
Helium FIM image of the {111} planes of iridium, 78 K. |
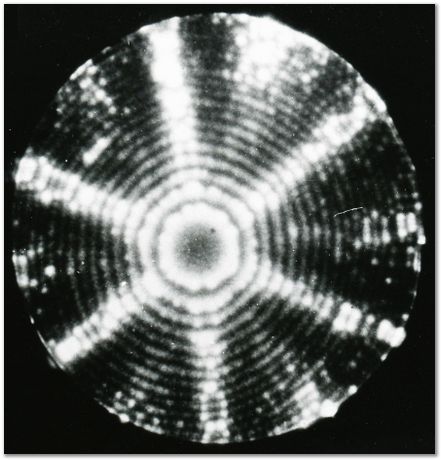
FIM image of the {111} planes of iridium. |
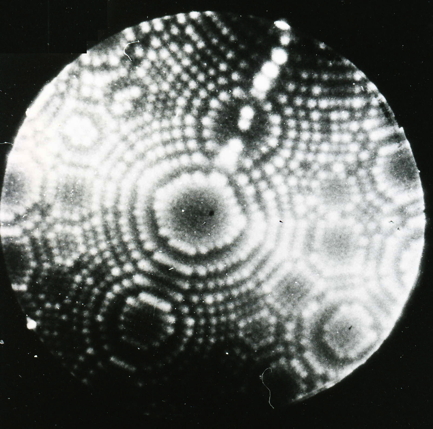
Helium FIM image of the {113} planes of iridium, 78 K. |