Lower bainite
Lower bainite has a microstructure and crystallographic
features which are very similar to those of upper bainite.
The major distinction is that cementite particles also
precipitate inside the plates of ferrite
Figure. There are,
therefore, two kinds of cementite precipitates: those which
grow from the carbon-enriched austenite which separates the
platelets of bainitic ferrite, and others which appear to
precipitate from supersaturated ferrite. These latter
particles exhibit the "tempering" orientation
relationship which is found when carbides precipitate during
the heat treatment of martensite, often described as the
Bagaryatski orientation relationship:
[0 0 1] Fe3C || [ -1 0 1]alpha
[1 0 0] Fe3C || [ 1 1 1]alpha
[0 1 0] Fe3C || [ -1 2 -1]alpha
|
The microstructure of lower bainite. Notice the precipitation of several variants of carbide particles within the plate of lower bainitic ferrite. Lower bainite otherwise also consists of fine platelets organised in sheaves, with each platelet separated partially by films of carbon-enriched retained austenite or carbides. After Bhadeshia and Edmonds, Metallurgical Transactions A, volume 10A (1979) 895-907.
|
The carbides in the ferrite need not always be cementite.
Depending on the chemical composition and the transformation
temperature, other transition carbides may precipitate first.
For example, in high-carbon steels containing more than
about 1 wt.% silicon (which retards cementite formation),
epsilon carbide is commonly observed to precipitate in the
bainitic ferrite.
In contrast to tempered martensite, the cementite particles
in lower bainite frequently precipitate in just one variant
of the orientation relationship (
Figure), such that they
form parallel arrays at about 60 ° to the axis of the
bainite plate. In tempered martensite, the carbides tend to
precipitate in Widmanstatten arrays. This peculiar mode of
precipitation in lower bainitic ferrite may arise because the
carbides nucleate at the ferrite/austenite interface, and
hence attempt to adopt a unique variant of the orientation
relationship, one which gives an optimum match to both the
austenite and ferrite with which they are in contact.
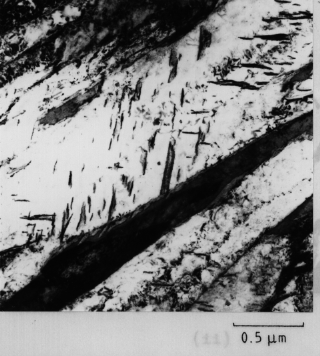
A transmission electron micrograph of lower bainite showing a single variant of carbide particles in each plate. Single variants tend to form when the driving force for cementite precipitation is small, i.e. in low carbon steels or at high temperatures where the carbon can escape rapidly from supersaturated ferrite. After Bhadeshia, Acta Metallurgica, volume 28 (1980) 1103-1114. |

|
Another plausible explanation is that the carbide
precipitation is influenced by the stresses associated with
the displacive growth of lower bainite. The effect would be
less pronounced during the tempering of martensite because
the driving force for carbide precipitation is larger.
The carbides in the lower bainite are extremely fine, just a
few nanometres thick and about 500 nm long. Because they
precipitate within the ferrite, a smaller amount of carbon is
partitioned into the residual austenite. This in turn means
that fewer and finer cementite particles precipitate between
the ferrite plates, when compared with an upper bainitic
microstructure. An important consequence is that lower
bainite is usually found to be much tougher than upper
bainite, in spite of the fact that it also tends to be
stronger. The coarse cementite particles in upper bainite are
notorious in their ability to nucleate cleavage cracks and
voids.

