19. In an Fe-C alloy, ferrite grows isothermally with
equilibrium at the interface such that
![]()
where ![]() is the concentration of carbon in ferrite
which is in equilibrium with austenite,
is the concentration of carbon in ferrite
which is in equilibrium with austenite, ![]() is the
corresponding concentration in austenite which is in equilibrium with
ferrite, D is the diffusivity of carbon in austenite, v is the
interface velocity and z is a coordinate defined
normal to the interface. The concentration gradient is evaluated at the
position of the interface.
is the
corresponding concentration in austenite which is in equilibrium with
ferrite, D is the diffusivity of carbon in austenite, v is the
interface velocity and z is a coordinate defined
normal to the interface. The concentration gradient is evaluated at the
position of the interface.
Explain separately the meanings of the left and right hand sides of this equation, and hence the origin of the equation.
How would similar conditions be derived for a ternary Fe-Mn-C alloy?
Hence describe two ways in which ferrite can grow in a ternary Fe-Mn-C alloy whilst maintaining local equilibrium at the interface, even though the diffusivities of Mn and C are different by many orders of magnitude.
Explain what is meant by the term paraequilibrium. Illustrate schematically an isothermal section of the paraequilibrium phase diagram for an Fe-Mn-C alloy where austenite and ferrite can coexist. The sketch should include a few tie-lines.
20. Describe the underlying causes of ferromagnetic hysteresis.
What parameters are used to define a hysteresis loop? How does the loop change with an increase in defect density and what is an anhysteretic loop? Describe the difference between soft and hard magnetic materials in terms of hysteresis loops.
Discuss the problem of hysteresis loss and the steps that can be taken to minimise it.
For low values of the magnetic induction B,
the hysteresis loop with tips (maxima and minima) at ![]() and
and
![]() is given by
is given by
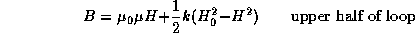
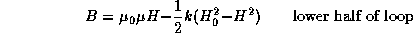
where k is a constant and H is the magnetic field.
(a) Sketch the form of this loop;
(b) derive expressions for the remanence and the coercive
field;
(c) show that the hysteresis loss per cycle of the field
is ![]() .
.
21. What is St. Venant's principle?
Describe qualitatively how the elastic stress distribution varies around a circular hole in a plate under uniaxial tension.
By analogy with the stress concentration around a hole, derive an approximate formula for the stress concentration at a notch, stating your assumptions.
A ![]() rosette of strain gauges is place on a
notched sample under tension. The strain gauges read
rosette of strain gauges is place on a
notched sample under tension. The strain gauges read
![]()
Given that strain gauge B is parallel to the applied force,
calculate the principal strains and their directions relative to
the applied force.
The rosette is placed a long way from the notch but in the same orientation. What are the angles between the strain gauges and the principal directions? What other technique might be used to characterise the strain in the sample?
22. What is crystallographic texture and how can it be measured experimentally? Describe various ways in which the texture generated in a sheet can be represented quantitatively, outlining the strengths and weaknesses of each representation.
Using a Wulff net or otherwise, draw an accurate stereogram
for a cubic close-packed crystal with ![]() at the centre
showing all poles of the form
at the centre
showing all poles of the form ![]() ,
, ![]() and
and
![]() .
.
Annealing twins in copper are related to the matrix copper
grains by reflections in ![]() planes. Show that a vector
planes. Show that a vector
![]() in a twin is related to a vector r in the
matrix by reflection across a plane whose normal is
n where
in a twin is related to a vector r in the
matrix by reflection across a plane whose normal is
n where
![]()
Determine ![]() if
if ![]() and
and
![]() and plot the pole corresponding to
and plot the pole corresponding to ![]() on your stereogram.
on your stereogram.
Hence sketch the ![]() pole figure for rolled
and annealed copper in which the matrix grains have a strong
pole figure for rolled
and annealed copper in which the matrix grains have a strong
![]() texture.
texture.
SECTION B
23. What is meant by a regular solution and how does it differ from ideal and real solutions? Describe how the regular solution model can be used to define phase diagrams. How can non-equilibrium phases be predicted?
The vapour pressure of manganese over an iron-manganese alloy at
1450 K is 0.417 mbar at a mole fraction of manganese of 0.6. The vapour
pressure of pure manganese at the same temperature is 0.635 mbar.
Calculate the following, assuming a regular solution model and that the
![]() function is independent of composition:
function is independent of composition:
(a) activity of manganese;
(b) activity coefficient of manganese;
(c) ![]() function;
function;
(d) heat of mixing.
24. Answer all of the following:
(a) What are the crystal structures of the two main
allotropic forms (![]() and
and ![]() ) of titanium, and which of these
two is stable at ambient temperature?
) of titanium, and which of these
two is stable at ambient temperature?
(b) Sketch the three main kinds of phase diagrams found
for titanium alloys given that some solutes stabilise ![]() , some
stabilise
, some
stabilise ![]() and others are neutral. On the diagram for
and others are neutral. On the diagram for ![]() stabilising elements, construct and justify a curve defining the locus of
the martensite-start temperature as a function of the solute
concentration.
stabilising elements, construct and justify a curve defining the locus of
the martensite-start temperature as a function of the solute
concentration.
(c) Why is the eutectoid reaction found in some titanium alloys so sluggish compared with the formation of pearlite in steels?
(d) Giving reasons, state typical applications for Ti-Pd and Ti-V-Al alloys.
25. What is meant by an energy gap ![]() in the electron
band structure of a material? Illustrating your answer with typical
values of
in the electron
band structure of a material? Illustrating your answer with typical
values of ![]() and schematic band structure diagrams. Discuss the
significance of the energy gap in determining the electrical
conductivity of a semiconductor, listing any other factors which may be
important.
and schematic band structure diagrams. Discuss the
significance of the energy gap in determining the electrical
conductivity of a semiconductor, listing any other factors which may be
important.
If the electrical conductivity of an intrinsic semiconductor is
proportional to ![]() , suggest a way of
measuring its energy gap.
, suggest a way of
measuring its energy gap.
Distinguish between a direct and indirect energy gap and explain how this affects optical transitions in semiconductors. How might the energy gap of a semiconductor be determined from its reflectivity spectrum?
The absorption edge of intrinsic germanium is measured at 300 K and found to be 1771 nm. What temperature rise will result in a 50% increase in its electrical conductivity?
26. Show that the stress ![]() acting on a plane defined
by its direction cosines
acting on a plane defined
by its direction cosines ![]() is given by
is given by
![]() where
where ![]() is the stress
tensor.
is the stress
tensor.
Derive an expression for the normal components of this
stress in terms of ![]() and
and ![]() . How may the
shear stresses be obtained?
. How may the
shear stresses be obtained?
A single crystal of copper is subjected to the stress
tensor
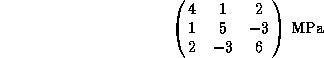
where the tensor axes are parallel to the crystallographic
axes. Which slip system involving the ![]() plane will
experience the highest shear stress? What is the magnitude of
this shear stress?
plane will
experience the highest shear stress? What is the magnitude of
this shear stress?
27. Discuss the factors which may limit the speed of the solid-liquid interface in solidification, distinguishing between diffusion-limited and collision-limited dynamics.
Define the solute partition coefficient for solidification; sketch how this coefficient varies with solidification speed. What is solute trapping? Why is it possible for the chemical potential of the solute to rise in this process? What are the thermodynamic and kinetic requirements for solute trapping?
For a given solidification speed, the degree of solute trapping may vary with crystal orientation; what is the origin of such an effect?
28. Figures 1a-c show Pourbaix diagrams plotted for 25oC for Zn, Ni and Ag, which can be used to address the following questions:
(a) Explain for each diagram what the lines labelled a and b represent and derive their equations in terms of voltage and pH.
(b) How do the diagrams relate to the position of each metal in the electrochemical series? Estimate values of Eo for each metal.
(c) Comment on the effect of pH and of competing reactions in circumstances where the metals are to be electrolytically extracted from 1 M solutions.
(d) All three metals are placed, without mutual contact, in a beaker of water at pH=7. Explain what would happen if the metal surfaces are bare, and for the case where the metal surfaces are in their ordinary states. How would the results change if the water is acidified to pH=1 and if oxygen is bubbled vigorously through the solution?
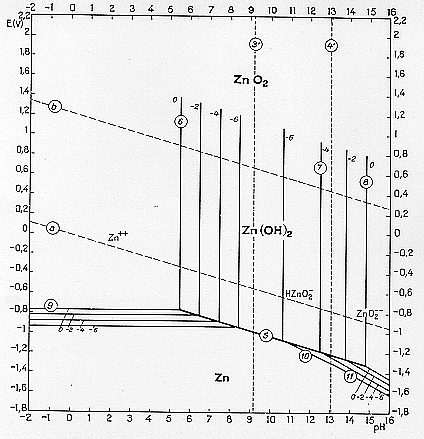 Figure 1a
Figure 1a
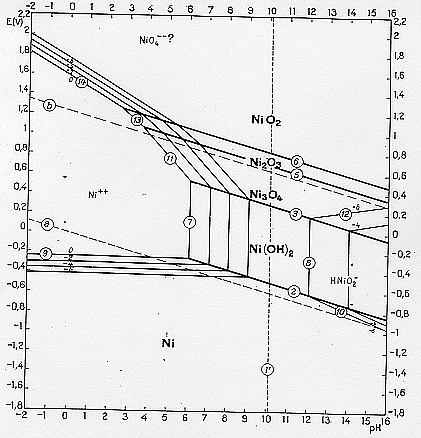 Figure 1b
Figure 1b
 Figure 1c
Figure 1c