
 |
Materials Algorithms Project
|
Jeevan Jaidi
Research Scholar (Ph. D),
Mechanical Engineering Department,
Indian Institute of Science,
Bangalore-560012,
INDIA.
E-mail: jaidi@mecheng.iisc.ernet.in
H. K. D. H. Bhadeshia
Phase Transformations Group,
Department of Materials Science and Metallurgy,
University of Cambridge,
Cambridge, U.K.
Added to MAP: May 2003.
Program to forecast the development of microstructure in the heat-affected zone of low-alloy steel weldments. Given the chemical composition and cooling conditions, it is possible to estimate the fractions of ferrite, pearlite, bainite and martensite) as a function of the austenite grain size and temperature.
| Language: | FORTRAN-90 |
| Product form: | Source code |
Complete program.
In fusion welding processes, the thermal cycle consists of heating and cooling periods. Within the heat-affected zone (HAZ), the formation of austenite on heating and its decomposition on cooling depends on the position with respect to the fusion boundary.
The method has been described in references 1 and 2, based on original theory in reference 3. Some of the equations in references 1,2 contain significant errors, and hence are reproduced in a corrected form in the presentation that follows. The units of a variety of empirical activation energies were also not stated in the original papers [1-3]; they are presented below in J/mole.
The austenite can, during cooling, transform into ferrite, pearlite, bainite and martensite. The start and finish temperatures of each of the phases are calculated as follows:

Austenite decomposition is characterised as follows:
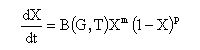
where X is the volume fraction of the transformation product at a given instant of time, G is the ASTM grain size number, and m and p are empirical constants related to the nucleation and growth model.
Austenite is stable at temperatures above the Ae3 line and is unstable below the Ae3 line. As the temperature drops below the Ae3, ferrite begins to form. The austenite-ferrite decomposition rate equation is given by:
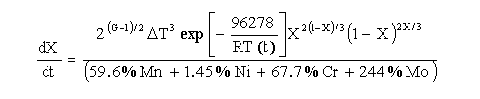
For temperatures belo eutectoid, and depending upon the cooling rate, the untransformed austenite will tend to decompose to pearlite. The austenite-pearlite decomposition rate is given by:
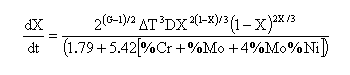
where D is the diffusion coefficient (concentrations of Cr and Mn expressed in weight percent. An error in the original equation published on MAP where carbon was mistakenly substituted for chromium, has been corrected both here an in the source code (courtesy of David Schroder, March 2014):
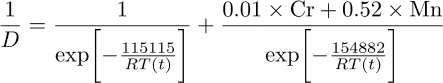
When the bainite-start temperaure is reached, the pearlite is in this empirical theory, assumed to continue to form bainite at a rate given by:
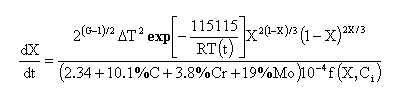
where f(X,Ci) is expressed by the following:
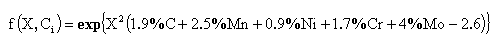
The remaining austenite is thenassumed to form martensite.
In the rate equations, the supercooling is defined with respect to the start-temperature of the phase concerned. The average austenite grain size in micro-meters is calculated numerically using the free grain growth model. This may not be appropriate once transformation has started. The ASTM grain size number is then calcuted from the calculated average grain size multiplied by 1.776 to allow for the conversion of two-dimensional measurements to three dimensions.
The time steps of the order of 10-4 s are required to integrate the austenite decomposition rate equation.
No information supplied.
Complete program.
The concentraion (%wt) of alloying elements and the polynomial coefficients for temperature vs time and average grain size vs time are the input data to be given in the file haz_input.txt. The following sequence is adapted for the concentration of alloying elements. No. Element C(1) C C(2) Ni C(3) Si C(4) V C(5) Mo C(6) W C(7) Mn C(8) Cr C(9) Cu C(10) P C(11) Al C(12) As C(13) Ti Coefficients of polynomial equation for temperature vs time A(1) A(2) A(3) A(4) A(5) Coefficients of polynomial equation for average grain size vs time B(1) B(2) B(3) See file haz_input.txt
See file haz_output.txt
None
ASTM grain size number, super cooling, heat-affected zone (HAZ), rate equation, austenite decomposition, ferrite, pearlite, bainite and martensite.
MAP originated from a joint project of the National Physical Laboratory and the University of Cambridge.