![[MAP Logo]](https://www.phase-trans.msm.cam.ac.uk/map/maplogo1.gif)
A.Y. Badmos and H.K.D.H. Bhadeshia, modified 2006 by M. Murugananth
Phase Transformations Group,
Department of Materials Science and Metallurgy,
University of Cambridge,
Cambridge, U.K.
To calculate free energy of mixing, configurational enthropy of mixing, enthalpy of mixing, and structural interfacial energy in mechanical alloying as functions of concentration, particle size and temperature.
The program is self-contained.
| Language: | FORTRAN |
| Product form: | Source code |
Molar entropy of mixing, Delta SM expressed as a function of atoms per particle is:-
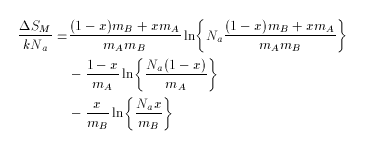
where Na is Avogadro's number, mA is atoms per powder particle of A, mB is atoms per particle of B, and x is the mole fraction of B.
Molar enthalpy of mixing, Delta HM, is expressed as:-
![]()
where Omega is the regular solution parameter, 2delta is the boundary thickness (two monolayer) and SV is grain boundary area per unit volume.
Molar interface energy, Delta HI, is expressed as:-
![]()
where Vm is the molar volume and sigma is the interface energy per unit area.
The molar free energy, Delta GM, is then expressed as:-
![]()
In the case of Omega > 0, the effect is appreciable only when the value of Omega is above about 100.
No information supplied.
None.
Complete program
Free energy, DELTAG, for different particle sizes (atoms per particle), M_A are:
Omega = 100, mole fraction, x=0.5, temperature, T=1000K
M_A=10^8, G=12.9669 J/mol M_A=10^2, G=12.9043 J/mol M_A=1, G= -5.435 KJ/mol.
Omega = 0, mole fraction, x=0.5, temperature, T=1000K
M_A=10^8, G=12.2617 J/mol M_A=10^2, G= -57.6181 J/mol M_A=1, G= -5.763 KJ/mol
Omega = -100, mole fraction, x=0.5, temperature, T=1000K
M_A=10^8, G=11.5564
M_A=10^2, G= -128.14 J/mol
M_A=1, G= -6.090 KJ/mol
None.
free energy of mixing, configurational enthropy of mixing, enthalpy of mixing, structural interfacial energy in mechanical alloying
Download modified source code (2006)
MAP originated from a joint project of the National Physical Laboratory and the University of Cambridge.
Top | Index | MAP Homepage ![]()