Stating assumptions, derive an equation to show how the measured diffusion coefficient depends on the grain size of a polycrystalline material.
What role do grain boundaries play in the performance of turbine blades used in the construction of aeroengines, and how may you minimise their deterimental effects?
For a unit area, the overall flux is the sum of that through the
lattice and that through the boundary:
Grain boundaries provide paths for easy diffusion and hence increase the rate of creep. The blades were at first polycrystalline, later to be replaced by directionally solidified blades so that the creep rate along the axis is reduced, and finally by single crystal turbine blades.
Stating reasons, describe metallic alloys and casting process which would be suitable for making the following cast components:
An engine block would be made out of an aluminium-12 wt% silicon
eutectic composition because this gives the minimum
melting temperature. The silicon which has a density of just 2.34
![]() , precipitates virtually as pure silicon. The resulting
expansion compensates for freezing contractions to give castings with
minimal porosity. The silicon is coarse and brittle. The addition of a
minute quantity of sodium (0.02 wt%) greatly refines the Si particles
giving a higher toughness.
, precipitates virtually as pure silicon. The resulting
expansion compensates for freezing contractions to give castings with
minimal porosity. The silicon is coarse and brittle. The addition of a
minute quantity of sodium (0.02 wt%) greatly refines the Si particles
giving a higher toughness.
The toy would be made by die-casting which involves the injection of a low-melting temperature alloy into a steel mould. Zn-4Al wt%, which is a eutectic composition, is a classic die-casting alloy melting at 419oC. The process is usually for non-structural components since the casting inevitably contains internal pores and because Zn does not have particularly good mechanical properties. However, productivity can be high and the surface finish is good.
A heat-resistant nickel base alloy cast using the lost wax process in which a wax model is surrounded by ceramic, the wax is then burnt off and the metal cast into the ceramic mould which is finally broken away to reveal the precise and beautiful casting.
|
Thickness / m
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Time / s | 1 | 4 | 9 | 16 | 25 | 36 | 49 | 64 |
Explain why does growth rate decreases as the particle becomes thicker.
The thickness varies with the square root of time (Fig. a), meaning that the process is diffusion-controlled.
From data book, the velocity ![]() is given by
is given by
The growth rate decreases with time (Figure). The physical reason why the
growth rate decreases with time is apparent from mass balance. As the
precipitate grows, the total amount of solute absorbed must equal that
depleted from the matrix. Thus, the diffusion distance
![]() is proportional to the precipitate size
is proportional to the precipitate size ![]() (Figure). As a
consequence, the concentration gradient decreases as the precipitate
thickens, causing a reduction in the growth rate.
(Figure). As a
consequence, the concentration gradient decreases as the precipitate
thickens, causing a reduction in the growth rate.
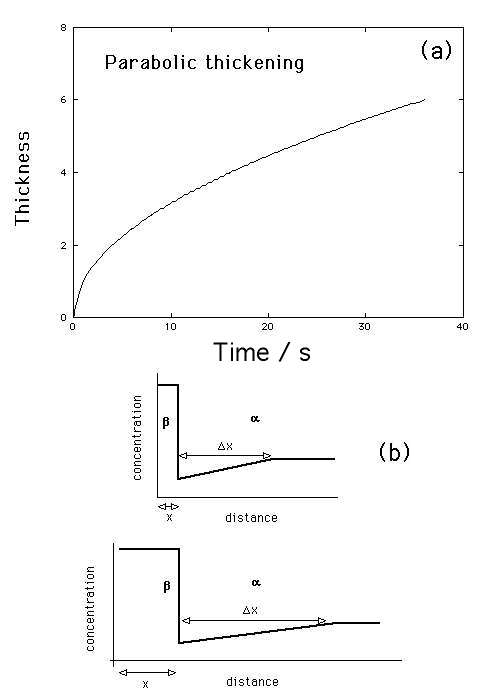
(a)
Parabolic thickening during one dimensional growth. (b) Increase in
diffusion distance as the precipitate thickens.