<--Previous Up Next-->
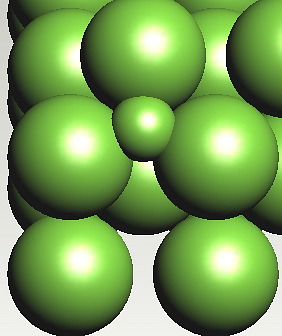
Possible position of carbon atom in a tetrahedral interstice in ferrite. The strain energy is greater when carbon is in a tetrahedral interstice, because the expansion is isotropic, unlike the octahedral case where the strain is tetragonal.
