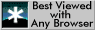
Worked Examples 1-4
Worked Examples 5-13
Worked Examples 14-20
Worked Examples 30-33
Explain separately the meanings of the left and right hand sides of this equation, and hence the origin of the equation.
How would similar conditions be derived for a ternary Fe-Mn-C alloy?
Hence describe two ways in which ferrite can grow in a ternary Fe-Mn-C alloy whilst maintaining local equilibrium at the interface, even though the diffusivities of Mn and C are different by many orders of magnitude.
Explain what is meant by the term paraequilibrium. Illustrate schematically an isothermal section of the paraequilibrium phase diagram for an Fe-Mn-C alloy where austenite and ferrite can coexist. The sketch should include a few tie-lines.
The boundary condition defined by the equation relates the interface velocity to the concentration gradient at the interface. The rate at which solute is partitioned as the interface moves ( i.e. left hand side) must equal that at which it is carried away by diffusion ( i.e. right hand side) if the compositions at the moving interface are to remain constant.
For a ternary alloy it would be necessary to satisfy two such equations
simultaneously, for each of the solutes:
Because
![]() these equations cannot in
general be simultaneously satisfied for the tie-line passing
through the alloy composition
these equations cannot in
general be simultaneously satisfied for the tie-line passing
through the alloy composition
![]() It is, however, possible to
choose other tie-lines which satisfy equation . If the tie-line
is such that
It is, however, possible to
choose other tie-lines which satisfy equation . If the tie-line
is such that
![]() (line
cd for alloy A of Figure 1a), then
(line
cd for alloy A of Figure 1a), then ![]() will become
very small, the driving force for carbon diffusion in effect
being reduced, so that the flux of carbon atoms is forced to
slow down to a rate consistent with the diffusion of manganese.
Ferrite forming by this mechanism is said to grow by a `
Partitioning, Local Equilibrium' (or PLE) mechanism, in
recognition of the fact that
will become
very small, the driving force for carbon diffusion in effect
being reduced, so that the flux of carbon atoms is forced to
slow down to a rate consistent with the diffusion of manganese.
Ferrite forming by this mechanism is said to grow by a `
Partitioning, Local Equilibrium' (or PLE) mechanism, in
recognition of the fact that
![]() can
differ significantly from
can
differ significantly from
![]() , giving
considerable partitioning and long-range diffusion of manganese into the
austenite (Coates, 1973c).
, giving
considerable partitioning and long-range diffusion of manganese into the
austenite (Coates, 1973c).
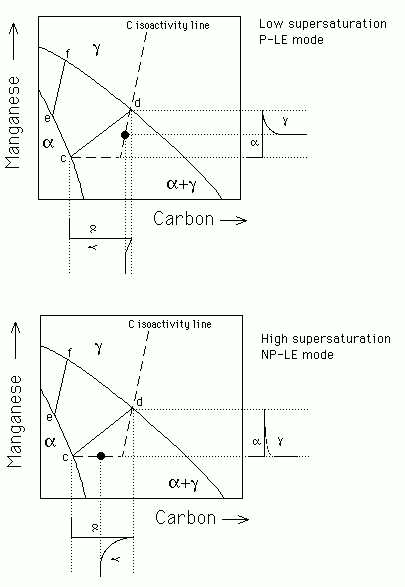
Figure 1: Schematic
isothermal sections of the Fe-Mn-C system, illustrating ferrite growth
occurring with local equilibrium at the
![]() interface.
(a) Growth at low supersaturations (P-LE) with bulk redistribution of
manganese, (b) growth at high supersaturations (NP-LE) with negligible
partitioning of manganese during transformation. The bulk alloy
compositions are designated by the symbol
interface.
(a) Growth at low supersaturations (P-LE) with bulk redistribution of
manganese, (b) growth at high supersaturations (NP-LE) with negligible
partitioning of manganese during transformation. The bulk alloy
compositions are designated by the symbol ![]() in each case.
in each case.
An alternative choice of tie-line could allow
![]() (line cd for alloy B
of Figure 1b), so that
(line cd for alloy B
of Figure 1b), so that ![]() is drastically increased
since only very small amounts of Mn are partitioned into the
austenite. The flux of manganese atoms at the interface correspondingly
increases and manganese diffusion can then keep pace with that of
carbon, satisfying the mass conservation conditions of
equation . The growth of ferrite in this manner is said to occur
by a `Negligible Partitioning, Local Equilibrium' (or NPLE) mechanism,
in recognition of the fact that the manganese content of the ferrite
approximately equals
is drastically increased
since only very small amounts of Mn are partitioned into the
austenite. The flux of manganese atoms at the interface correspondingly
increases and manganese diffusion can then keep pace with that of
carbon, satisfying the mass conservation conditions of
equation . The growth of ferrite in this manner is said to occur
by a `Negligible Partitioning, Local Equilibrium' (or NPLE) mechanism,
in recognition of the fact that the manganese content of the ferrite
approximately equals
![]() , so that little if any
manganese partitions into austenite.
, so that little if any
manganese partitions into austenite.
Paraequilbrium is a constrained equilibrium (Figure 2). It occurs at temperatures where the diffusion of substitutional solutes is not possible within the time scale of the experiment. Nevertheless, intersitials may remain highly mobile. Thus, in a steel, manganese does not partition between the ferrite and austenite, but subject to that constraint, the carbon redistributes until it has the same chemical potential in both phases.
Therefore, the tie-lines in the phase diagram are all virtually parallel to the carbon axis, since Mn does not partition between ferrite and austenite.
Figure 2: Paraequilibrium
phase diagram.
(ii) Sketch the three main kinds of phase diagrams found for
titanium alloys given that some solutes stabilise ![]() , some
stabilise
, some
stabilise ![]() and others are neutral. On the diagram for
and others are neutral. On the diagram for ![]() stabilising elements, construct and justify a curve defining the locus of
the martensite-start temperature as a function of the solute
concentration.
stabilising elements, construct and justify a curve defining the locus of
the martensite-start temperature as a function of the solute
concentration.
(iii) Why is the eutectoid reaction found in some titanium alloys so sluggish compared with the formation of pearlite in steels?
(iv) Giving reasons, state typical applications for Ti-Pd and Ti-V-Al alloys.
The three types of phase diagrams are illustrated below, for ![]() ,
,
![]() and neutral elements which lead to a eutectoid phase diagram.
The locus of
and neutral elements which lead to a eutectoid phase diagram.
The locus of ![]() temperatures is indicated as a dashed line, with
sufficient undercooling to account for strain energy and solute
supersaturation.
temperatures is indicated as a dashed line, with
sufficient undercooling to account for strain energy and solute
supersaturation.
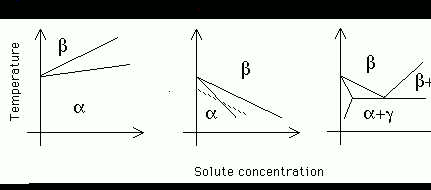
Figure 3: Phase
diagrams for titanium.
The eutectoid reaction, for example in the Ti-Cu alloys, involves the solid-state diffusion of substitutional solutes which diffuse at a far smaller rate than intersitial carbon in iron. Consequently, it is frequently possible to avoid the eutectoid reaction by cooling rapidly.
The addition of a small amount (![]() wt%) of paladium to titanium
makes it much more corrosion resistant in acid. This is because the
hydrogen evolution reaction is less polarised (hydrogen evolution is
easier), causing the cathodic reaction curve to intersect the cathodic
reaction curve in the passive region, leading to a smaller corrosion
current (Figure 3).
wt%) of paladium to titanium
makes it much more corrosion resistant in acid. This is because the
hydrogen evolution reaction is less polarised (hydrogen evolution is
easier), causing the cathodic reaction curve to intersect the cathodic
reaction curve in the passive region, leading to a smaller corrosion
current (Figure 3).
Ti-6Al-4V is a classic ![]() alloy with high strength
combined with a low density. The
alloy with high strength
combined with a low density. The ![]() phase is harder and enhances
the overall strength, the aluminium helping to stabilise
phase is harder and enhances
the overall strength, the aluminium helping to stabilise ![]() and
lower the density. However, 6Al needs to be balanced by the 4V which is
an
and
lower the density. However, 6Al needs to be balanced by the 4V which is
an ![]() stabiliser. The alloy is widely used in sheet and forgings
for the aerospace industries.
stabiliser. The alloy is widely used in sheet and forgings
for the aerospace industries.
(a) martensite, Widmanstätten ferrite and bainite all occur in the form of thin plates;
(b) Widmanstätten ferrite grows at a rate which is much smaller than the speed of sound in the metal;
(c) an Fe-30Ni wt% martensite is weak compared with martensite in Fe-0.2C wt% alloy;
(d) alloying elements have a much greater effect on the kinetics of a reconstructive transformation when compared with the corresponding effect on displacive transformations..
Widmanstätten ferrite occurs at temperatures where diffusionless transformation is thermodynamically impossible. Thus, although the change in crystal structure is accomplished by a deformation, the displacements occur at a rate controlled by the diffusion of carbon in the austenite ahead of the interface.
Carbon causes a much greater degree of hardening than any substitutional solute. This is because it leads to a tetragonal distortion of the b.c.c. structure; a tetragonal strain can interact with all components of the stress field of a dislocation. Substitutional solutes cause isotropic strains which can only interact with the small hydrostatic component of stress associated with edge dislocations.
The effect on displacive transformations is thermodynamic alone. On reconstructive transformations the solute may partition between the parent and product phases by a process involving diffusion. This will have a further kinetic effect on reconstructive transformations.
Given that the Bain strain, which changes the face-centered cubic austenite lattice into that of body-centered cubic martensite is an invariant-line strain, explain why the observed shape deformation accompanying martensitic transformation is an invariant-plane strain.
Why is the lattice-invariant deformation unnecessary when austenite transforms by a martensitic mechanism to a hexagonal close-packed lattice?
Why is martensite hard in steels but not so in iron or in non-ferrous metals and alloys?
Note that it is not correct to state that the transformation must occur at high speeds or at low temperatures, that it requires rapid quenching or that martensite is hard.
The phenomenological theory of
martensite crystallography solves this problem (Figure 4). The
Bain strain converts the structure of the parent phase into that of the
product phase. When combined with an appropriate rigid body rotation,
the net homogeneous lattice deformation ![]() is an
invariant-line strain (step
is an
invariant-line strain (step ![]() to
to ![]() in Figure 4). However, the
observed shape deformation is an invariant-plane strain
in Figure 4). However, the
observed shape deformation is an invariant-plane strain ![]() (step
(step ![]() to
to ![]() in Figure 4), but this gives the wrong crystal structure.
If a second homogeneous shear
in Figure 4), but this gives the wrong crystal structure.
If a second homogeneous shear ![]() is combined with
is combined with
![]() (step
(step ![]() to
to
![]() ), then the correct structure is obtained but the wrong shape since
), then the correct structure is obtained but the wrong shape since
The theory explains all the observed features of the martensite crystallography. The orientation relationship is predicted by deducing the rotation needed to change the Bain strain into an invariant-line strain. The habit plane does not have rational indices because the amount of lattice-invariant deformation needed to recover the correct the macroscopic shape is not usually rational. The theory predicts a substructure in plates of martensite (either twins or slip steps) as is observed experimentally. The transformation goes to all the trouble of ensuring that the shape deformation is macroscopically an invariant-plane strain because this reduces the strain energy when compared with the case where the shape deformation might be an invariant-line strain.
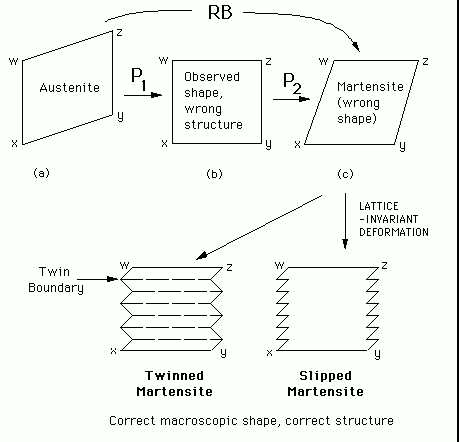
Figure 4: The
phenomenological theory of martensite crystallography.
Notice that in the case of the fcc-hcp transformation, the lattice
deformation (Bain Strain) itself is an invariant-palne strain, i.e. the
motion of Shockley partials on the alternate close-packed planes.
B![]() so a lattice invariant deformation is not needed. There
are no transformation twins or slip steps to be found.
so a lattice invariant deformation is not needed. There
are no transformation twins or slip steps to be found.
The carbon atom in a b.c.c. lattice causes a tetragonal strain because it resides in an irregular octahedral hole, whose axes are given by the cell edge and two orthogonal face diagonals. A tetragonal strain can interact with both shear and hydrostatic components of stress. Thus, there is a strong interaction with both screw and edge dislocations. By contrast, substitutional solutes (and carbon in austenite) only cause isotropic volume changes which can only interact with the hydrostatic component of stress from edge dislocations, i.e. a weak interaction.
(ii) Explain the cause of precipitate-free zones and how they might be avoided.
(iii) Why are aluminium-silicon alloys good as casting alloys? How might their microstructures be improved?
It is possible, at large driving forces, for precipitates to nucleate homogeneously. However, it is more common for nucleation to occur heterogeneously because there is a gain in energy as the defect on which the particle forms is partly or wholly eliminated.
Vacancies are a prominent nucleation site for many precipitates, particularly in aluminium alloys where the concentration of vacancies tends to be high. However, there is a vacancy denuded zone next to a grain boundary (which is a sink for vacancies), Figure 5. There will, therefore, be a precipitate-free zone (PFZ) adjacent to a grain boundary.
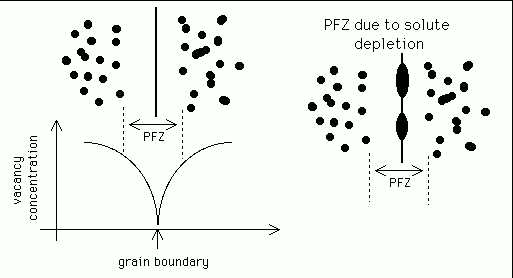
Figure 5:
Precipitate-free zones.
An alternative reason for PFZ formation is that nucleation happens first on grain boundaries which are themselves potent nucleation sites. These precipitates drain the adjacent matrix from solute and hence a solute depleted PFZ arises, with coarse particles at the boundaries.
Precipitate-free zones can be eliminated by a two-stage heat treatment
where nucleation is induced homogeneously at a low temperature, and the
precipitates are then allowed to grow at a higher temperature (Figure 6).
Thus, for Al-6Zn-3Mg age-hardening alloy, GP zones are used as nuclei
for the ![]() (
(![]() phase.
phase.
twostage22294400Figure 6: Two-stage heat treatment
Aluminium casting alloys closely resemble cast irons.
Aluminium-12 wt% silicon eutectic compositions are frequently used
because this gives the minimum melting temperature. The silicon which
has a density of just 2.34
![]() , precipitates virtually as
pure silicon. The resulting expansion compensates for freezing
contractions to give castings with minimal porosity.
, precipitates virtually as
pure silicon. The resulting expansion compensates for freezing
contractions to give castings with minimal porosity.
The silicon is coarse and brittle. The addition of a minute quantity of sodium (0.02 wt%) greatly refines the Si particles giving a higher toughness. It does so by removing P; AlP is a good nucleant for Si so its removal allows solidification to occur at a higher undercooling, where the nucleation rate can be larger.
(b) Explain why the most popular titanium alloy contains both aluminium and vanadium as alloying additions.
(c) Giving one example, explain what is meant by solute trapping.
(d) Distinguish between martensite, lower bainite, upper bainite and Widmanstätten ferrite.
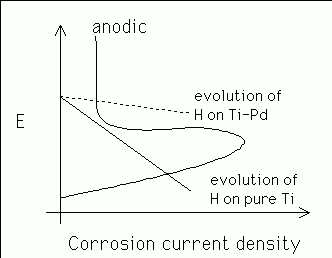
Figure 6: Corrosion of
Ti and Ti-Pd.
Aluminium reduces the density of Ti and stabilizes and strengthens the
![]() phase while vanadium provides a greater amount of more ductile
phase while vanadium provides a greater amount of more ductile
![]() phase during hot-working.
phase during hot-working.
Solute is said to be trapped when its chemical potential increases on
transfer across the moving interface. It occurs when the interface
velocity becomes comparable to the diffusion velocity ![]() wheere
wheere ![]() is the interatomic jump distance. Examples: rapid
solidification, martensitic transformation (C in Fe), paraequilibrium (Mn
in ferrite).
is the interatomic jump distance. Examples: rapid
solidification, martensitic transformation (C in Fe), paraequilibrium (Mn
in ferrite).
Martensitic transformation is diffusionless. Bainite grows without diffusion but because it forms at relatively high temperatures, the carbon can rapidly (a second or so) escape into the residual austenite. It may then precipitate as cementite, giving an upper bainite microstructure. If the bainite transformation temperature is reduced then the carbon escapes less rapidly so that it is possible for some of it to precipitate inside the ferrite plates, giving a lower bainite microstructure (Fig. 6.12, Honeycombe & Bhadeshia). Widmanstätten ferrite grows by a displacive paraequilibrium mechanism involving the diffusion of carbon. It thus grows at a rate which is controlled by the diffusion of carbon in the austenite ahead of the interface. Furthermore, there is little driving force available at the temperatures at which Widmanstätten ferrite grows so two plates have to grow together in a self-accommodating manner in order to reduce the overall strain energy. This is why it optically appears to be in the form of a thin wedge, since the component plates have slightly different habit planes.
Worked Examples 1-4
Worked Examples 5-13
Worked Examples 14-20
H.K.D.H. Bhadeshia