
Worked Examples 1-4
Worked Examples 5-13
Worked Examples 14-20
Worked Examples 21-29
Upper bainite in conventional steels consists of a mixture of bainitic ferrite and cementite. How can this cementite be eliminated from the microstructure?
When the precipitation of cementite is prevented, the microstructure obtained by isothermal transformation in the bainite temperature range consists of just bainitic ferrite and carbon-enriched retained austenite. State four potential advantages of this microstructure from the point of view of toughness and strength.
One difficulty with the mixed microstructure of bainitic ferrite and retained austenite is that the austenite, when present in large quantities, is unstable and hence forms high-carbon, brittle martensite. Explain three ways in which this problem can be eliminated.
Silicon has an incredibly small solubility in cementite. Therefore, increasing the silicon concentration of a steel to a value greater than about 1.5 wt% ensures the absence of cementite in upper bainite. Aluminium and to a lesser extent chromium, have the same effect. Note that silicon has a similar effect in cast irons. (20%)
The six potential advantages (only four required in the answer) of the microstructure can be listed as follows: (40%)
The problem of unstable retained austenite arises because the
amount of bainite that can form is limited by the ![]() curve on
the phase diagram. Therefore, the three ways all relate to the
curve on
the phase diagram. Therefore, the three ways all relate to the
![]() curve, as follows: (40%)
curve, as follows: (40%)
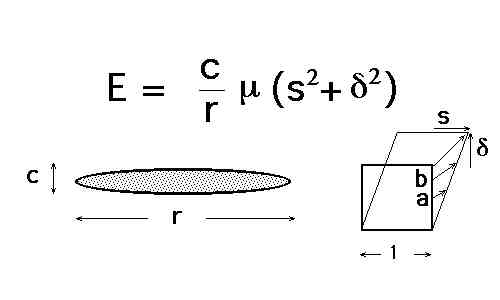
Explain what is meant by the term paraequilibrium. Illustrate schematically an isothermal section of the paraequilibrium phase diagram for an Fe-Mn-C alloy where austenite and ferrite can coexist. The sketch should include tie-lines.
Why does the austenite![]() ferrite phase field converge to a point
when the carbon concentration becomes zero?
ferrite phase field converge to a point
when the carbon concentration becomes zero?
Define in thermodynamic terms, what is meant when an element is said to be trapped during transformation.
During the paraequilibrium growth of ferrite in an Fe-Mn-C alloy, which of the elements is trapped in the ferrite, and which in austenite?
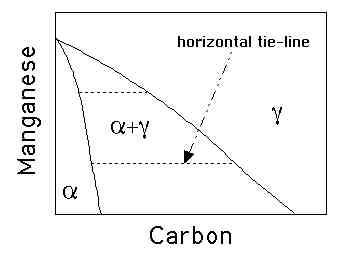
Therefore, the tie-lines in the phase diagram are all virtually parallel to the carbon axis, since Mn does not partition between ferrite and austenite. (20%)
At zero carbon concentration, the austenite and ferrite have
exactly the same chemical composition since Mn does not
redistribute during transformation. Therefore, there is a unique
concentration at which the two phases have equal free energy,
they are in equilibrium. (10%)
An element is said to be trapped when its chemical potential increases on transfer across the moving interface. (10%)
The manganese is trapped in the ferrite since its chemical potential increases on transfer across the interface, the iron is trapped in the austenite for the same reason. Manganese is an austenite stabiliser so its equilibrium concentration in austenite should be greater than that in ferrite. (20%)
How would the transformation manifest in an electron diffraction pattern?
Describe any other diffusionless transformation possible in titanium alloys and state the observed orientation relationship.
How would you represent the temperature below which diffusionless transformation becomes thermodynamically possible on a phase diagram for a binary titanium alloy?
The
![]() transformation is reversible and diffusionless but is
not martensitic in the classical sense since there is no invariant-plane strain shape
deformation. However, it does involve the coordinated motion of atoms.
transformation is reversible and diffusionless but is
not martensitic in the classical sense since there is no invariant-plane strain shape
deformation. However, it does involve the coordinated motion of atoms.
The body-centred cubic (bcc) crystal structure of ![]() can be imagined as the stacking of
can be imagined as the stacking of
![]() planes in an
planes in an
![]() stacking sequence. Note that these planes are
not close-packed in the bcc structure. The
stacking sequence. Note that these planes are
not close-packed in the bcc structure. The
![]() transformation
occurs by the passage of a longitudinal displacement wave along
transformation
occurs by the passage of a longitudinal displacement wave along ![]() which causes the
which causes the ![]() and
and ![]() planes to collapse into each other, leaving the
planes to collapse into each other, leaving the ![]() planes unaffected. The stacking
sequence thus changes to
planes unaffected. The stacking
sequence thus changes to
![]() in which the
in which the ![]() planes have twice the density
of atoms as the
planes have twice the density
of atoms as the ![]() planes. The
planes. The
![]() stacking is consistent with a
stacking is consistent with a ![]() a hexagonal crystal structure with a
a hexagonal crystal structure with a
![]() . The atoms in the
. The atoms in the ![]() plane have a trigonal coordination which is similar to that in
graphite and the bonding becomes partly covalent. (60%)
plane have a trigonal coordination which is similar to that in
graphite and the bonding becomes partly covalent. (60%)
This leads to an increase in the electrical resistivity. The longitudinal displacement waves are responsible for the streaking in the electron diffraction patterns. (10%)
Quenching the ![]() phase leads to the formation of h.c.p.
phase leads to the formation of h.c.p. ![]() martensite. The orientation relationship is with the most densly
packed planes parallel and the corresponding most densly packed
directions parallel (the relationship is actually irrational, so
the stated orientation is approximate).
martensite. The orientation relationship is with the most densly
packed planes parallel and the corresponding most densly packed
directions parallel (the relationship is actually irrational, so
the stated orientation is approximate).
The temperature is represented by the ![]() curve.
curve.
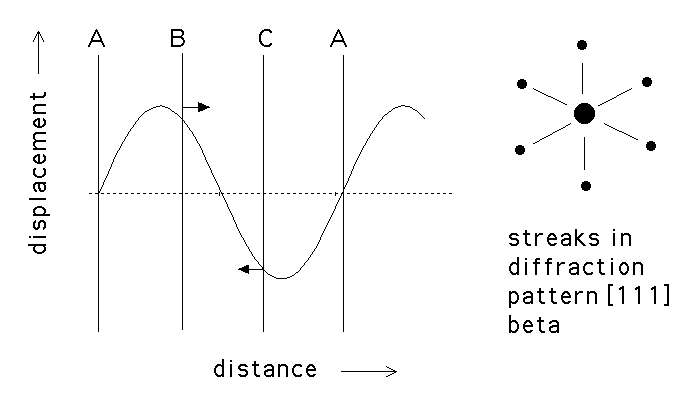
(a) Displacement
wave associated with the
![]() to
to ![]() transformation. The
transformation. The ![]() planes are unaffected since they lie at the
nodes. (b) Streaks in the electron diffraction pattern during the
planes are unaffected since they lie at the
nodes. (b) Streaks in the electron diffraction pattern during the ![]() transformation.
transformation.
Give three features of martensite that distinguish it from a reconstructive transformation such as allotriomorphic ferrite. Explain how you might characterise each of these features using experiments.
Giving reasons, explain which of the following deformations is an invariant-plane strain
Show diagrammatically that it is not possible to transform austenite into body-centred cubic martensite by a deformation which is an invariant plane strain.
Why is martensite hard in steels but not so in iron or in other non-ferrous metals and alloys?
A martensitic transformation is achieved by a deformation of the parent crystal structure; it therefore leads to a shape deformation which has a large shear component, and which can be detected by polishing the parent phase prior to transformation. The shape deformation causes surface tilts which can be measured using atomic force microscopy, interference optical microscopy or by the deflection of fiducial marks. The formation of allotriomorphic ferrite is not accompanied by any shear strain, simply a small volume change which does not deflect any fiducial marks.
Martensitic transformations are diffusionless and hence the measured composition of martensite must always be the same as that of the parent phase. The chemical composition of allotriomorphic ferrite corresponds to its equilibrium composition and hence in general will differ from that of the parent phase. The chemical composition can be measured using energy dispersive X-ray microanalysis, the atom-probe technique and X-ray diffraction.
The interface between martensite and the parent phase must be glissile, it must be able to move without diffusion. The glissile character can be established by electron microscopy in which the Burgers vectors of the interfacial dislocations are measured and shown to lie out of the plane of the interface (although for pure screws they may lie in the interface plane). There are no such requirements for the structure of the interface between allotriomorphic ferrite and austenite (it may be sessile or glissle, and will always require diffusion in order to translate).
Note that it is not correct to state that the transformation must occur at high speeds or at low temperatures, that it requires rapid quenching or that martensite is hard. Martensite can be soft, slow, and can take place at high temperatures.
Suppose that the austenite is represented as a sphere with its unit cell
edges denoted by the vectors ![]() with
with ![]() , as illustrated
in a,b. The Bain strain changes the sphere into an ellipsoid of
revolution about
, as illustrated
in a,b. The Bain strain changes the sphere into an ellipsoid of
revolution about ![]() . There are no lines in the
. There are no lines in the
![]() plane which are undistorted. However, it is possible to
find lines such as
plane which are undistorted. However, it is possible to
find lines such as ![]() and
and ![]() are undistorted by the deformation, but
are rotated to the new positions
are undistorted by the deformation, but
are rotated to the new positions ![]() and
and ![]() . Since they are
rotated by the Bain deformation they are not invariant-lines. In fact,
the Bain strain does not produced an invariant-line strain. It can be
converted into an invariant-line strain by adding a rigid body rotation
as illustrated in c. The rotation reorients the
. Since they are
rotated by the Bain deformation they are not invariant-lines. In fact,
the Bain strain does not produced an invariant-line strain. It can be
converted into an invariant-line strain by adding a rigid body rotation
as illustrated in c. The rotation reorients the ![]() lattice
but has no effect on its crystal structure. The effect of the rotation is
to make one of the original undistorted lines (in this case
lattice
but has no effect on its crystal structure. The effect of the rotation is
to make one of the original undistorted lines (in this case
![]() ) invariant so that the total effect BR of the Bain strain
B and the rotation R is indeed an invariant-line strain. This is
the reason why the observed irrational orientation relationship
(KS/NW type) differs from that implied by the Bain strain. The rotation
required to generate convert B to an ILS precisely predicts the
observed orientation from the Bain orientation.
) invariant so that the total effect BR of the Bain strain
B and the rotation R is indeed an invariant-line strain. This is
the reason why the observed irrational orientation relationship
(KS/NW type) differs from that implied by the Bain strain. The rotation
required to generate convert B to an ILS precisely predicts the
observed orientation from the Bain orientation.
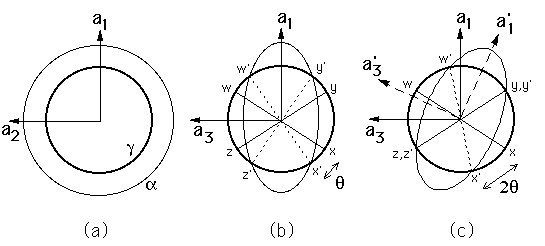
(a) and (b) show the
effect of the Bain strain on austenite, which when undeformed is
represented as a sphere of diameter ![]() in three-dimensions. The
strain transforms it to an ellipsoid of revolution. (c) shows the
invariant-line strain obtained by combining the Bain strain with a rigid
body rotation.
in three-dimensions. The
strain transforms it to an ellipsoid of revolution. (c) shows the
invariant-line strain obtained by combining the Bain strain with a rigid
body rotation.
It is also apparent from (c) that there is no possible rotation
which would convert B into an invariant-plane strain because
there is no rotation capable of making two of the non-parallel
undistorted lines into invariant-lines. Thus, it is impossible to
convert austenite into ![]() martensite by a strain which is an
invariant-plane strain. A corollary to this statement is that the two
crystals cannot ever be joined at an interface which is fully coherent
and stress-free.
martensite by a strain which is an
invariant-plane strain. A corollary to this statement is that the two
crystals cannot ever be joined at an interface which is fully coherent
and stress-free.
The carbon atom in a b.c.c. lattice causes a tetragonal strain because it resides in an irregular octahedral hole, whose axes are given by the cell edge and two orthogonal face diagonals. A tetragonal strain can interact with both shear and hydrostatic components of stress. Thus, there is a strong interaction with both screw and edge dislocations. By contrast, substitutional solutes (and carbon in austenite) only cause isotropic volume changes which can only interact with the hydrostatic component of stress from edge dislocations, a weak interaction.
Describe the roles of aluminium and vanadium in Ti-6Al-4V alloy. Why should the use of this alloy in an aeroengine be limited to temperatures less than about 400oC?
Al reduces density, stabilises and strengthens
![]() while vanadium provides a greater a mount of the more
ductile
while vanadium provides a greater a mount of the more
ductile ![]() phase for hot-working. This alloy is very commonly
used because of its strength (1100 MPa), creep resistance at
300 , fatigue resistance and castability.
Common titanium alloys have low densities and can be creep
resistant. However, titanium fires can occur when titanium
components accidentally rub against other metals when the
temperature of the environment is greater than about 400.
phase for hot-working. This alloy is very commonly
used because of its strength (1100 MPa), creep resistance at
300 , fatigue resistance and castability.
Common titanium alloys have low densities and can be creep
resistant. However, titanium fires can occur when titanium
components accidentally rub against other metals when the
temperature of the environment is greater than about 400.
Explain what is meant by the term paraequilibrium. Illustrate schematically an isothermal section of the paraequilibrium phase diagram for an Fe-Mn-C alloy where austenite and ferrite can coexist. The sketch should include tie-lines with an explanation of how you construct them.
Explain the mechanism of the transformation in steels, including a description of the factors that determine its growth rate and shape.
Which solute is said to be trapped in the product phase during transformation of austenite in a Fe-Mn-C alloy, in the following cases:
Paraequilibrium is a constrained equilibrium. It occurs at temperatures where the diffusion of substitutional solutes is not possible within the time scale of the experiment. Nevertheless, interstitials may remain highly mobile. Thus, in a steel, manganese does not partition between the ferrite and austenite, but subject to that constraint, the carbon redistributes until it has the same chemical potential in both phases.
Therefore, the tie-lines in the phase diagram are all virtually parallel to the carbon axis, since Mn does not partition between ferrite and austenite.
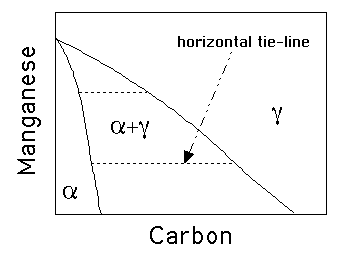
Widmanstatten ferrite grows by a displacive paraequilibrium mechanism involving the diffusion of carbon. It thus grows at a rate which is controlled by the diffusion of carbon in the austenite ahead of the interface. Furthermore, there is little driving force available at the temperatures at which the ferrite grows so two plates have to grow together in a self-accommodating manner in order to reduce the overall strain energy. This is why it optically appears to be in the form of a thin wedge, since the component plates have slightly different habit planes.
Prove that the solubility of solute in an aluminium alloy
containing copper is greater when the matrix is in equilibrium with
a metastable precipitate such as GP1, when compared with the case
where it is in equilibrium with the stable precipitate CuAl![]() .
.
Assuming that the Al-Cu
solution can be described by a regular solution thermodynamic
model, show that the solubility ![]() of solute in the matrix varies
as follows:
of solute in the matrix varies
as follows:
Would you expect the copper atoms to cluster or to order at low temperatures?
The solubility of solute in the matrix ![]() is
therefore of importance. This solubility cannot be defined in isolation
- it depends on the phase with which the
is
therefore of importance. This solubility cannot be defined in isolation
- it depends on the phase with which the ![]() is in equilibrium
with, . In the Al-Cu system, the stable precipitate is
CuAl
is in equilibrium
with, . In the Al-Cu system, the stable precipitate is
CuAl![]() but because it is difficult to nucleate, metastable GP1 zones
form first. Thus, the free energy curve for GP1 zones is located above
that for CuAl
but because it is difficult to nucleate, metastable GP1 zones
form first. Thus, the free energy curve for GP1 zones is located above
that for CuAl![]() in b. The common tangent construction shows that
this leads to an increase in the solubility of copper in
in b. The common tangent construction shows that
this leads to an increase in the solubility of copper in ![]() when it is in equilibrium with GP1 zones. Another interpretation is
that a greater undercooling is required before GP1 zones can
precipitate.
when it is in equilibrium with GP1 zones. Another interpretation is
that a greater undercooling is required before GP1 zones can
precipitate.
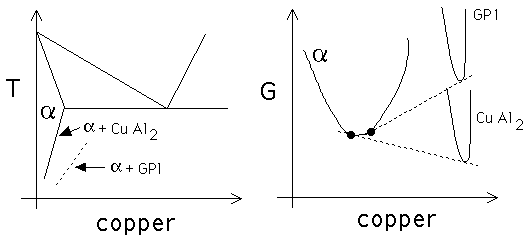
(a) The solubility
of solute in ![]() is larger when it is in equilibrium with GP1 zones
compared with when it is in equilibrium with CuAl
is larger when it is in equilibrium with GP1 zones
compared with when it is in equilibrium with CuAl![]() . (b) This can be
justified using free energy diagrams.
. (b) This can be
justified using free energy diagrams.
Since ![]() is positive, so is the enthalpy of mixing
is positive, so is the enthalpy of mixing ![]() . The free energy versus composition plot will therefore have
two minima at low temperatures. The minimum at lower concentrations
gives the solubility in matrix, it is safe to assume
. The free energy versus composition plot will therefore have
two minima at low temperatures. The minimum at lower concentrations
gives the solubility in matrix, it is safe to assume ![]() .
.
The miscibility gap at any temperature can be determined by the
usual common tangent construction on the free energy curve with
the two minima. Noting that the regular solution model has symmetry
about
![]() , the compositions corresponding to the common tangent
construction can in this special case be obtained by setting
, the compositions corresponding to the common tangent
construction can in this special case be obtained by setting