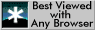
Worked Examples 1-4
Worked Examples 5-13
Worked Examples 14-20
Worked Examples 21-29
Upper bainite in conventional steels consists of a mixture of bainitic ferrite and cementite. How can this cementite be eliminated from the microstructure?
When the precipitation of cementite is prevented, the microstructure obtained by isothermal transformation in the bainite temperature range consists of just bainitic ferrite and carbon-enriched retained austenite. State four potential advantages of this microstructure from the point of view of toughness and strength.
One difficulty with the mixed microstructure of bainitic ferrite and retained austenite is that the austenite, when present in large quantities, is unstable and hence forms high-carbon, brittle martensite. Explain three ways in which this problem can be eliminated.
Silicon has an incredibly small solubility in cementite. Therefore, increasing the silicon concentration of a steel to a value greater than about 1.5 wt% ensures the absence of cementite in upper bainite. Aluminium and to a lesser extent chromium, have the same effect. Note that silicon has a similar effect in cast irons. (20%)
The six potential advantages (only four required in the answer) of the microstructure can be listed as follows: (40%)
The problem of unstable retained austenite arises because the
amount of bainite that can form is limited by the ![]() curve on
the phase diagram. Therefore, the three ways all relate to the
curve on
the phase diagram. Therefore, the three ways all relate to the
![]() curve, as follows: (40%)
curve, as follows: (40%)
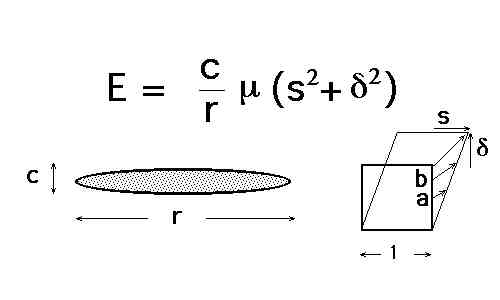
Explain what is meant by the term paraequilibrium. Illustrate schematically an isothermal section of the paraequilibrium phase diagram for an Fe-Mn-C alloy where austenite and ferrite can coexist. The sketch should include tie-lines.
Why does the austenite![]() ferrite phase field converge to a point
when the carbon concentration becomes zero?
ferrite phase field converge to a point
when the carbon concentration becomes zero?
Define in thermodynamic terms, what is meant when an element is said to be trapped during transformation.
During the paraequilibrium growth of ferrite in an Fe-Mn-C alloy, which of the elements is trapped in the ferrite, and which in austenite?
Therefore, the tie-lines in the phase diagram are all virtually parallel to the carbon axis, since Mn does not partition between ferrite and austenite. (20%)
At zero carbon concentration, the austenite and ferrite have
exactly the same chemical composition since Mn does not
redistribute during transformation. Therefore, there is a unique
concentration at which the two phases have equal free energy,
they are in equilibrium. (10%)
An element is said to be trapped when its chemical potential increases on transfer across the moving interface. (10%)
The manganese is trapped in the ferrite since its chemical potential increases on transfer across the interface, the iron is trapped in the austenite for the same reason. Manganese is an austenite stabiliser so its equilibrium concentration in austenite should be greater than that in ferrite. (20%)
How would the transformation manifest in an electron diffraction pattern?
Describe any other diffusionless transformation possible in titanium alloys and state the observed orientation relationship.
How would you represent the temperature below which diffusionless transformation becomes thermodynamically possible on a phase diagram for a binary titanium alloy?
The
![]() transformation is reversible and diffusionless but is
not martensitic in the classical sense since there is no invariant-plane strain shape
deformation. However, it does involve the coordinated motion of atoms.
transformation is reversible and diffusionless but is
not martensitic in the classical sense since there is no invariant-plane strain shape
deformation. However, it does involve the coordinated motion of atoms.
The body-centred cubic (bcc) crystal structure of ![]() can be imagined as the stacking of
can be imagined as the stacking of
![]() planes in an
planes in an
![]() stacking sequence. Note that these planes are
not close-packed in the bcc structure. The
stacking sequence. Note that these planes are
not close-packed in the bcc structure. The
![]() transformation
occurs by the passage of a longitudinal displacement wave along
transformation
occurs by the passage of a longitudinal displacement wave along ![]() which causes the
which causes the ![]() and
and ![]() planes to collapse into each other, leaving the
planes to collapse into each other, leaving the ![]() planes unaffected. The stacking
sequence thus changes to
planes unaffected. The stacking
sequence thus changes to
![]() in which the
in which the ![]() planes have twice the density
of atoms as the
planes have twice the density
of atoms as the ![]() planes. The
planes. The
![]() stacking is consistent with a
stacking is consistent with a ![]() a hexagonal crystal structure with a
a hexagonal crystal structure with a
![]() . The atoms in the
. The atoms in the ![]() plane have a trigonal coordination which is similar to that in
graphite and the bonding becomes partly covalent. (60%)
plane have a trigonal coordination which is similar to that in
graphite and the bonding becomes partly covalent. (60%)
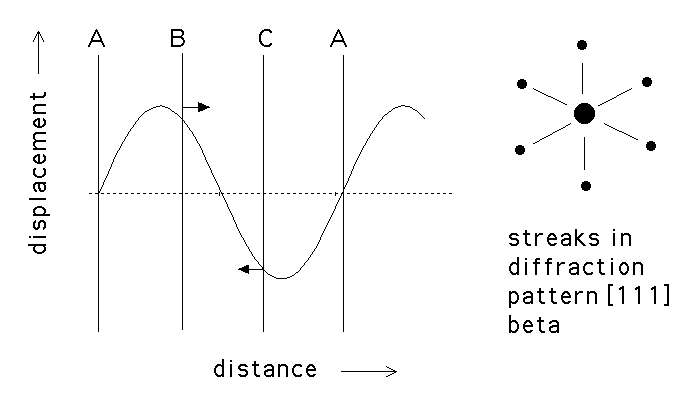
This leads to an increase in the electrical resistivity. The longitudinal displacement waves are responsible for the streaking in the electron diffraction patterns. (10%)
omega237144 350: (a) Displacement
wave associated with the
![]() to
to ![]() transformation. The
transformation. The ![]() planes are unaffected since they lie at the
nodes. (b) Streaks in the electron diffraction pattern during the
planes are unaffected since they lie at the
nodes. (b) Streaks in the electron diffraction pattern during the ![]() transformation.
transformation.
Quenching the ![]() phase leads to the formation of h.c.p.
phase leads to the formation of h.c.p. ![]() martensite. The orientation relationship is with the most densly
packed planes parallel and the corresponding most densly packed
directions parallel (the relationship is actually irrational, so
the stated orientation is approximate).
martensite. The orientation relationship is with the most densly
packed planes parallel and the corresponding most densly packed
directions parallel (the relationship is actually irrational, so
the stated orientation is approximate).
The temperature is represented by the ![]() curve.
curve.