The crystal structure of austenite consists of a cubic-F lattice with a motif of an iron atom located at each lattice point. The structure is often losely referred to as face-centred cubic or cubic close-packed.

|
The crystal structure of austenite consists of a cubic-F lattice with a motif of an iron atom located at each lattice point. The structure is often losely referred to as face-centred cubic or cubic close-packed. |
|
This figure shows two adjacent Cubic-F unit cells of austenite. | 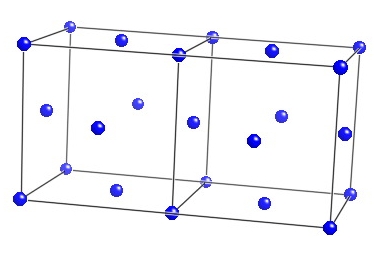 |
|
This figure shows two adjacent Cubic-F unit cells of austenite, with a body-centred tetragonal (b.c.t.) unit cells of austenite highlighted by colouring some of the lattice points in red. Notice that the body-centred cell is simply another representation of austenite. The relationship between the Cubic-F and b.c.t. cells is known as the Bain Correspondence. It is important because an appropriate compression applied along the z-axis together with a uniform expansion along the x- and y-axes would convert the b.c.t. cell of austenite into the body-centred cubic or body-centred tetragonal cells of martensite, bainite or Widmanstatten ferrite. | 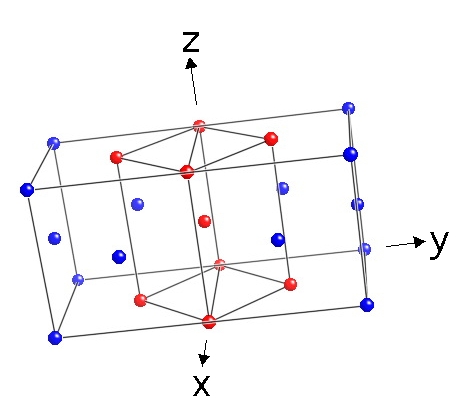 |
| PT Group Home | Materials Algorithms |

|

|