After some discussion, it was accepted in 1925 that the colours of thin films of oxide are interference effects (U. R. Evans, Proceedings of the Royal Society of London. Series A, Containing Papers of a Mathematical and Physical Character, Vol. 107, No. 742 (Feb. 2, 1925), pp. 228-237). See also G. Tammann, Zeitsch. Anorg. Chem., Vol. 111, 1920, p. 78.
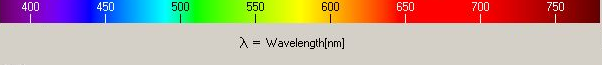
When light of a wavelength λ is reflected from metal covered with an oxide film, it is eliminated by destructive intereference if the thickness of the oxide is 0.25λ. This is because the reflected beam will then have a path difference with the incident beam of 0.5λ, thus leading to destructive interference.

Blue light has a wavelength of about 450 nm and red has a wavelength of about 725 nm. If white light is incident on the oxide, and the latter appears blue, the implication is that the red component is eliminated, meaning that the oxide layer is about 0.25×725 nm thick.
The colours of oxide films left on steel which has been placed in a temperature gradient range from brown, mauve, indigo, pale blue, steel colour, pink-lavender, and pale steel blue in order from the cold to the hot end (Evans, 1925).
The colours observed in the photographs below are consistent with the tempering temperature and indicate an oxide film thickness of about 200 nm.
Of course, if the oxide layers become thick then they become opaque and interferance through reflection from the metal surface is no longer possible.
The colour changes in this case are due first to ther formation of yellow dextrins in the crust, followed by caramelisation. Dextrins are formed by the breakdown of starch. Caramelisation is due to the oxidation of sugar. Photographs courtesy of Rosie Ward.
 Stage 1 |
 Stage 2 |
 Final colour |
|
| PT Group Home | Materials Algorithms |