A superalloy is a metallic alloy which can be used at high temperatures, often in excess of 0.7 of the absolute melting temperature. Creep and oxidation resistance are the prime design criteria. Superalloys can be based on iron, cobalt or nickel, the latter being best suited for aeroengine applications.
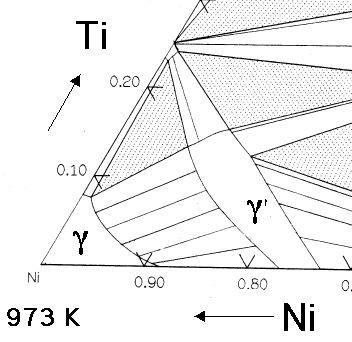
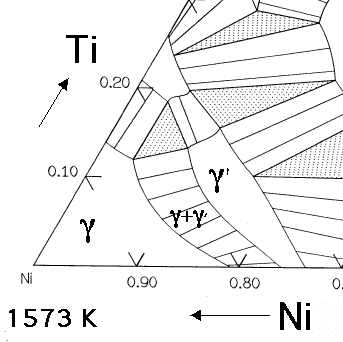
The essential solutes in nickel based superalloys are aluminium and/or titanium, with a total concentration which is typically less than 10 atomic percent. This generates a two-phase equilibrium microstructure, consisting of gamma (γ) and gamma-prime (γ'). It is the γ' which is largely responsible for the elevated-temperature strength of the material and its incredible resistance to creep deformation. The amount of γ' depends on the chemical composition and temperature, as illustrated in the ternary phase diagrams below.

|

|
The Ni-Al-Ti ternary phase diagrams show the γ and γ' phase field. For a given chemical composition, the fraction of γ' decreases as the temperature is increased. This phenomenon is used in order to dissolve the γ' at a sufficiently high temperature (a solution treatment) followed by ageing at a lower temperature in order to generate a uniform and fine dispersion of strengthening precipitates.
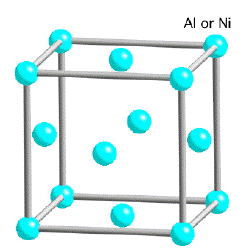
The γ-phase is a solid solution with a cubic-F lattice and a random distribution of the different species of atoms. Cubic-F is short for face-centred cubic.
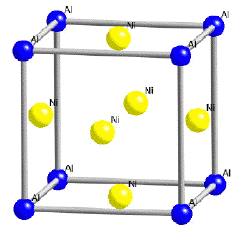
By contrast, γ' has a cubic-P (primitive cubic) lattice in which the nickel atoms are at the face-centres and the aluminium or titanium atoms at the cube corners. This atomic arrangement has the chemical formula Ni3Al, Ni3Ti or Ni3(Al,Ti). However, as can be seen from the (γ+γ')/γ' phase boundary on the ternary sections of the Ni, Al, Ti phase diagram, the phase is not strictly stoichiometric. There may exist an excess of vacancies on one of the sublattices which leads to deviations from stoichiometry; alternatively, some of the nickel atoms might occupy the Al sites and vice-versa. In addition to aluminium and titanium, niobium, hafnium and tantalum partition preferentially into γ'.
|
|
| Crystal structure of γ | Crystal structure of γ' |
The γ phase forms the matrix in which the γ' precipitates. Since both the phases have a cubic lattice with similar lattice parameters, the γ' precipitates in a cube-cube orientation relationship with the γ. This means that its cell edges are exactly parallel to corresponding edges of the γ phase. Furthermore, because their lattice parameters are similar, the γ' is coherent with the γ when the precipitate size is small. Dislocations in the γ nevertheless find it difficult to penetrate γ', partly because the γ' is an atomically ordered phase. The order interferes with dislocation motion and hence strengthens the alloy.
The small misfit between the γ and γ' lattices is important for two reasons. Firstly, when combined with the cube-cube orientation relationship, it ensures a low γ/γ' interfacial energy. The ordinary mechanism of precipitate coarsening is driven entirely by the minimisation of total interfacial energy. A coherent or semi-coherent interface therefore makes the microstructure stable, a property which is useful for elevated temperature applications.
The magnitude and sign of the misfit also influences the development of microstructure under the influence of a stress at elevated temperatures. The misfit is said to be positive when the γ' has a larger lattice parameter than γ. The misfit can be controlled by altering the chemical composition, particularly the aluminium to titanium ratio. A negative misfit stimulates the formation of rafts of γ', essentially layers of the phase in a direction normal to the applied stress. This can help reduce the creep rate if the mechanism involves the climb of dislocations across the precipitate rafts.
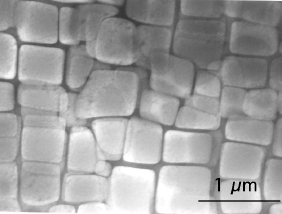
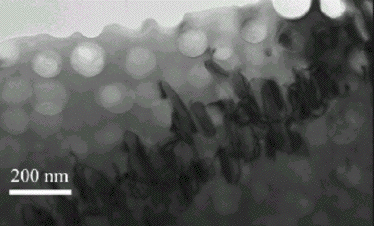
The transmission electron micrographs shown below illustrate the large fraction of γ', typically in excess of 0.6, in turbine blades designed for aeroengines, where the metal experiences temperatures in excess of 1000oC. Only a small fraction (0.2) of γ' is needed when the alloy is designed for service at relatively low temperatures (750oC) and where welding is used for fabrication.
|
|
|
Transmission electron micrograph showing a large fraction of cuboidal γ' particles in a γ matrix. Ni-9.7Al-1.7Ti-17.1Cr-6.3Co-2.3W at%. Hillier, Ph.D. Thesis, University of Cambridge, 1984. |
Transmission electron micrograph showing a small fraction of spheroidal γ' prime particles in a γ matrix. Ni-20Cr-2.3Al-2.1Ti-5Fe-0.07C-0.005 B wt%. Also illustrated are M23C6 carbide particles at the grain boundary running diagonally from bottom left to top right. |
The strength of most metals decreases as the temperature is increased, simply because assistance from thermal activation makes it easier for dislocations to surmount obstacles. However, nickel based superalloys containing γ', which essentially is an intermetallic compound based on the formula Ni3(Al,Ti), are particularly resistant to temperature.
Ordinary slip in both γ and γ' occurs on the {111}<110>. If slip was confined to these planes at all temperatures then the strength would decrease as the temperature is raised. However, there is a tendency for dislocations in γ' to cross-slip on to the {100} planes where they have a lower anti-phase domain boundary energy. This is because the energy decreases with temperature. Situations arise where the extended dislocation is then partly on the close-packed plane and partly on the cube plane. Such a dislocation becomes locked, leading to an increase in strength. The strength only decreases beyond about 600oC whence the thermal activation is sufficiently violent to allow the dislocations to overcome the obstacles.
To summarise, it is the presence of γ' which is responsible for the fact that the strength of nickel based superalloys is relatively insensitive to temperature.
|
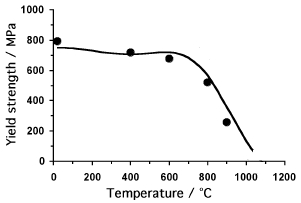
The yield strength of a particular superalloy containing only about 20% of γ'. The points are measured and the curve is a theoretical prediction. Notice how the strength is at first insensitive to temperature. |
|
|
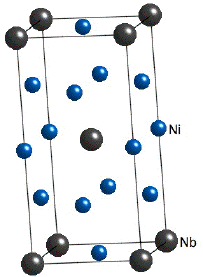
When greater strength is required at lower temperatures (e.g. turbine discs), alloys can be strengthened using another phase known as γ''. This phase occurs in nickel superalloys with significant additions of niobium (Inconel 718) or vanadium; the composition of the γ'' is then Ni3Nb or Ni3V. The particles of γ'' are in the form of discs with (001)γ''||{001}γ and [100]γ''||<100>γ The crystal structure of γ'' is based on a body-centred tetragonal lattice with an ordered arrangement of nickel and niobium atoms. Strengthening occurs therefore by both a coherency hardening and order hardening mechanism. The lattice parameters of γ'' are approximately a=0.362 nm and c=0.741 nm |
|
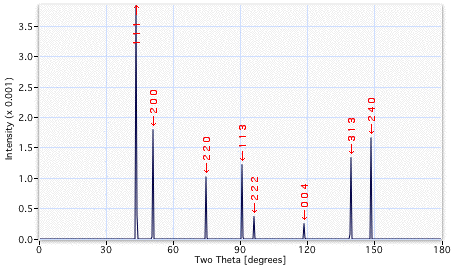
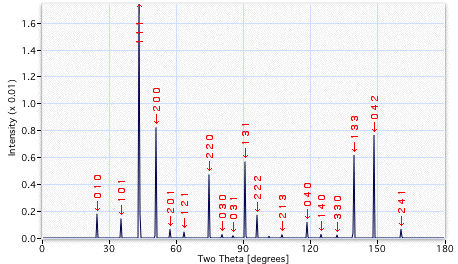
A comparison of the diffraction patterns indicated below reveals many more peaks from the γ'. The additional reflections are quite weak in intensity. They arise because the γ' lattice is primitive cubic, which means that planes such as {100} give rise to diffracted intensity, whereas the reflections from the corresponding {100} planes of γ have zero intensity (destructive interference with the {200} planes). The additional reflections from the γ' prime are termed superlattice reflections and are weak because they depend on the difference in scattering power between the Ni and Al atoms.
|
X-ray diffraction pattern from γ, for a particular set of diffraction conditions. |
|
X-ray diffraction pattern from γ', for a particular set of diffraction conditions. |
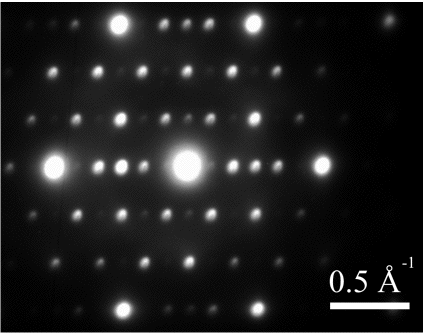
The figures below show a superimposed electron diffraction pattern from γ, γ' and M23C6 carbide. The γ and γ' phases have their cubic-lattice edges perfectly aligned.
 |
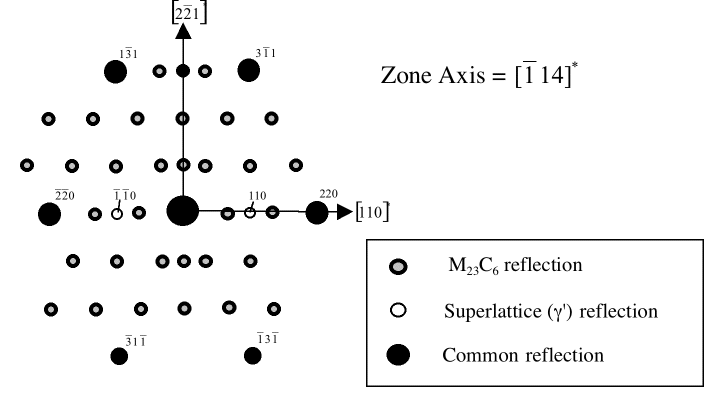 |
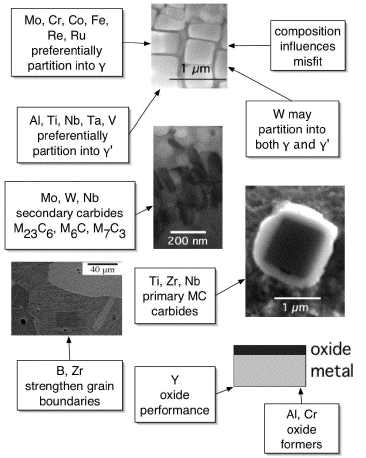
Commercial superalloys contain more than just Ni, Al and Ti. Chromium and aluminium are essential for oxidation resistance small quantities of yttrium help the oxide scale to cohere to the substrate. Polycrystalline superalloys contain grain boundary strengthening elements such as boron and zirconium, which segregate to the boundaries. The resulting reduction in grain boundary energy is associated with better creep strength and ductility when the mechanism of failure involves grain decohesion.
There are also the carbide formers (C, Cr, Mo, W, C, Nb, Ta, Ti and Hf). The carbides tend to precipitate at grain boundaries and hence reduce the tendency for grain boundary sliding.
Elements such as cobalt, iron, chromium, niobium, tantalum, molybdenum, tungsten, vanadium, titanium and aluminium are also solid-solution strengtheners, both in γ and γ'.
There are, naturally, limits to the concentrations that can be added without inducing precipitation. It is particularly important to avoid certain embrittling phases such as Laves and Sigma. There are no simple rules governing the critical concentrations; it is best to calculate or measure the appropriate part of a phase diagram.
| Alloying element effects in nickel based superalloys. The "M" in M23C6 stands for a mixture of metal atoms. Click on chart to enlarge. |
 |
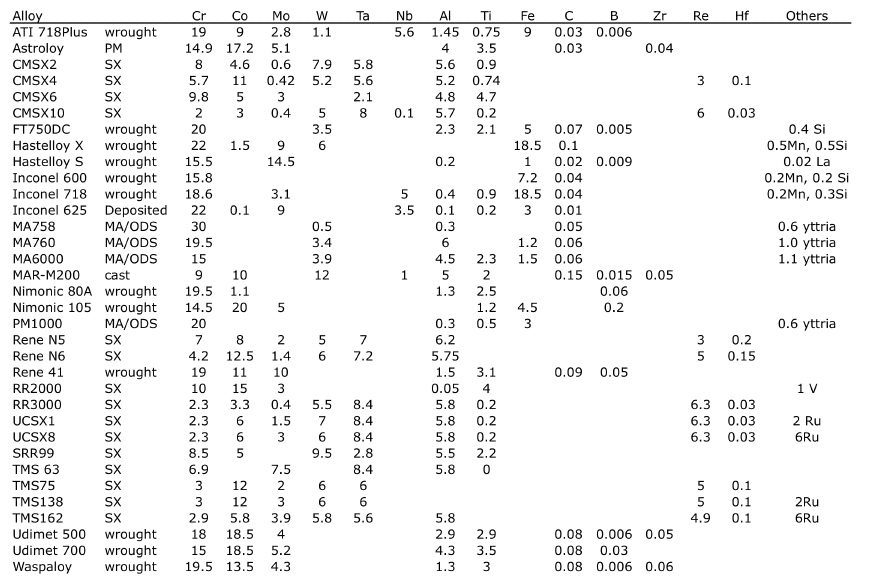 |
| Nominal chemical compositions, wt%. MA/ODS ≡ mechanically alloyed, oxide dispersion-strengthened.
PM ≡ powder metallurgical origin. The alloy names are proprietary. SX ≡ single crystal. |
The single-crystal superalloys are often classified into first, second and third generation alloys. The second and third generations contain about 3 wt% and 6wt% of rhenium respectively. Rhenium is a very expensive addition but leads to an improvement in the creep strength. It is argued that some of the enhanced resistance to creep comes from the promotion of rafting by rhenium, which partitions into the γ and makes the lattice misfit more negative. Atomic resolution experiments have shown that the Re occurs as clusters in the γ phase. It is also claimed that rhenium reduces the overall diffusion rate in nickel based superalloys.
The properties of superalloys deteriorate if certain phases known as the topologically close-packed (TCP) phases precipitate. In these phases, some of the atoms are arranged as in nickel, where the close-packed planes are stacked in the sequence ...ABCABC.. However, although this sequence is maintained in the TCP phases, the atoms are not close-packed, hence the adjective 'topologically'. TCP phases include σ and μ. Such phases are not only intrinsically brittle but their precipitation also depletes the matrix from valuable elements which are added for different purposes. The addition of rhenium promotes TCP formation, so alloys containing these solutes must have their Cr, Co, W or Mo concentrations reduced to compensate. It is generally not practical to remove all these elements, but the chromium concentration in the new generation superalloys is much reduced. Chromium does protect against oxidation, but oxidation can also be prevented by coating the blades.
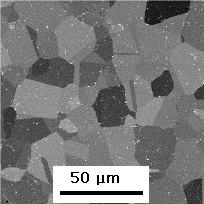
The solution heat treatment temperature determines not only the amount of γ' that dissolves, but also the grain size of the γ. The size becomes coarser if all the γ' is dissolved, since there is then no pinning effect of the precipitate particles on the movement of the γ/γ boundaries. The picture on the left has been heat treated at a sub-solvus temperature, that on the right at a super-solvus temperature. (Image courtesy of R. J. Mitchell). The The three-dimensional shape of the grains has recently been determined by Michael Uchic. |
|
|
Oxide dispersion strengthened superalloys can be produced starting from alloy powders and yttrium oxide, using the mechanical alloying process. The yttria becomes finely dispersed in the final product. It is also a very stable oxide, making the material particularly suitable for elevated temperature applications. However, mechanical alloying is a very difficult process so such alloys have limited applications. A transmission electron micrograph showing the oxide dispersion in a mechanically-alloyed nickel based superalloy is shown below.
 |
ODS alloy MA6000 |
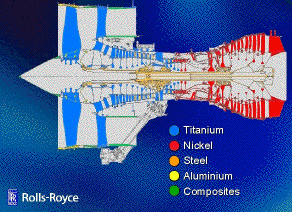
A major use of nickel based superalloys is in the manufacture of aeroengine turbine blades. A single-crystal blade is free from γ/γ grain boundaries. Boundaries are easy diffusion paths and therefore reduce the resistance of the material to creep deformation. The directionally solidified columnar grain structure has many γ grains, but the boundaries are mostly parallel to the major stress axis; the performance of such blades is not as good as the single-crystal blades. However, they are much better than the blade with the equiaxed grain structure which has the worst creep life.
One big advantage of the single-crystal alloys over conventionally cast polycrystalline superalloys is that many of the grain boundary strengthening solutes are removed. This results in an increase in the incipient melting temperature (i.e., localised melting due to chemical segregation). The single-crystal alloys can therefore be heat treated to at temperatures in the range 1240-1330°C, allowing the dissolution of coarse γ' which is a remanent of the solidification process. Subsequent heat treatment can therefore be used to achieve a controlled and fine-scale precipitation of γ'. The primary reason why the first generation of single-crystal superalloys could be used at higher temperatures than the directionally solidified ones, was because of the ability to heat-treat the alloys at a higher temperature rather than any advantage due to the removal of grain boundaries. A higher heat-treatment temperature allows all the γ' to be taken into solution and then by aging, to precipitate in a finer form.
Superalloy blades are used in aeroengines and gas turbines in regions where the temperature is in excess of about 400oC, with titanium blades in the colder regions. This is because there is a danger of titanium igniting in special circumstances if its temperature exceeds 400oC.
 |
 |
 |
 |
Single crystal |
Directionally solidified |
Equiaxed polycrystalline |
Engine materials (source: Michael Cervenka) |
| This movie has kindly been provided for educational purposes by Professor Hongbiao Dong of Leicester University. The simulation shows only one crystal makes it through the spiral selector so that the liquid above the selector solidifies as a single crystals as the assembly is lowered through a temperature gradient. |
Turbine blades are attached to a disc which in turn is connected to the turbine shaft. The properties required for an aeroengine discs are different from that of a turbine, because the metal experiences a lower temperature. The discs must resist fracture by fatigue. Discs are usually cast and then forged into shape. They are polycrystalline.
An internal combustion engine generally uses a stoichiometric ratio of air to fuel. A turbocharger is a device to force more air into the engine, allowing a correspondingly greater quantity of fuel to be burned in each stroke. This boosts the power output of the engine.
The turbocharger consists of two components, a turbine which is driven by exhaust gases from the engine. This in turn drives an air pump which forces more air into the engine. The typical rate of spin is 100-150,000 rotations per minute. Because the turbocharger is driven by exhaust gasses, it gets very hot and needs to be oxidation resistant and strong.
 | Turbocharger of nickel based superalloy Inconel 713C, Ni-2Nb-12.5Cr-4.2Mo-0.8Ti-6.1Al-0.12C-0.012B-0.1Zr wt%. |
The superalloys contain reactive elements such as aluminium and titanium. It is necessary therefore to melt the alloys under vaccum, with the added advantage that detrimental trace elements are removed by evaporation. Vaccum induction melting is commonly used because the inductive stirring encourages homogenisation and helps expose more of the liquid to the melt-vaccum interface. This in turn optimises the removal of undesirable gases and volatile impurities.
|
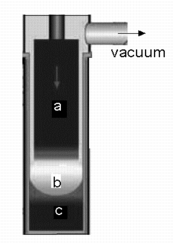
Many alloys are then vaccum arc remelted in order to achieve a higher purity and better solidification microstructure. The ingot is made an electrode (a). An arc burns in the vacuum, thereby heating the front end of the electrode. Droplets are formed which then trickle through the vacuum and become purified. The molten metal is contained by a water-cooled copper mould. There is a liquid pool (b) where further purification occurs by the floatation of solid impurities. The solidified metal (c) has a desirable directional-microstructure. |
|
The diagram for electroslag refining looks similar to that for vacuum arc remelting, except that the melt pool is covered by a 10 cm thick layer of slag (lime, alumina and flourite). The ingot is again an electrode in contact with the slag. The slag has a high electrical resistivity and hence melts, the temperature being in excess of the melting point of the metal electrode. The tip of the electrode melts, allowing metal to trickle through the slag into the liquid sump at the bottom. This refines the alloy.
It is common for alloys destined for critical applications to go through two or more of these melting processes.
Nickel based superalloy blades are generally made using an investment casting process. A wax model is made, around which a ceramic is poured to make the mould. The wax is removed from the solid ceramic and molten metal poured in to fill the mould. The actual process is more complicated because of the intricate shape of the blade, with its cooling channels and other features.
More information on nickel based superalloys.
Photograph of "superalloy", courtesy Franck Tancret.
I am grateful to Sammy Tin for arranging access to several of the micrographs.
 5th edition published 2024 |
 Free download |
 Free download |
 Available |
 Free download Published 2021 |
 Published, 2023 |
 Published 2022 |
 October 8th, 2024 |
| PT Group Home | Materials Algorithms |