
Hydrides in a Zr alloy
H. K. D. H. Bhadeshia
Zr-2.5Nb wt% alloys are used in certain nuclear reactors to contain fuel and heavy water (D2O); this is because zirconium has a low neutron-capture cross-section and is relatively (though not entirely) corrosion resistant in water-cooled reactors. The niobium confers strength and enhances corrosion resistance. Deuterium is an isotope of hydrogen; corrosion reactions introduce some hydrogen into the Zr alloy, resulting in the precipitation of embrittling hydrides.
There are two kinds of hydrides. δ-hydride ZrH1.6 has a cubic-F lattice whereas γ-hydride is face-centred tetragonal with a composition ZrH. Under appropriate conditions the δ may transform into γ.
The micrographs shown below have been provided for teaching purposes courtesy of Professor W. Murray Small of Pennsylvania State University. Details are published in J. H. Root, W. M. Small, D. Khatamian and O. T. Woo, Acta Materialia 51 (2003) 2041.

Zr-25.Nb wt% alloy showing slender particles of gamma-hydride in an alpha-Zr matrix.
|
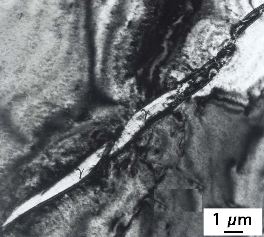
Zr-25.Nb wt% alloy showing slender particles of gamma-hydride in an alpha-Zr matrix.
|
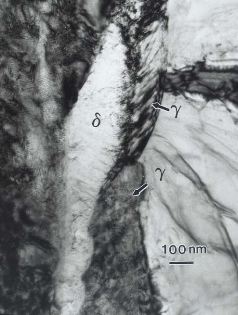
Transmission electron micrograph of a Zr-2.5Nb wt% sample showing delta and gamma hydrides in a matrix of alpha Zr.
|
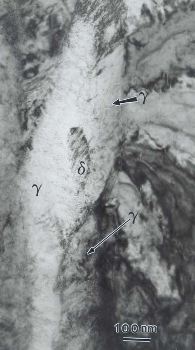
As in the previous micrograph, but observed after a year. Much of the delta has decomposed into gamma hydride.
|






