
Niobium Carbonitrides
Crystal Structure of Niobium Carbide
Niobium carbide has a cubic-F lattice with a motif of Nb at 0,0,0, and carbon at 0,0,0.5. The carbide is not strictly stoichiometric but is represented here as such, with a lattice parameter of 4.4691 nm.
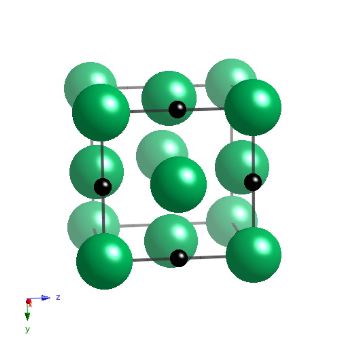
Crystal structure of NbC in perspective. |
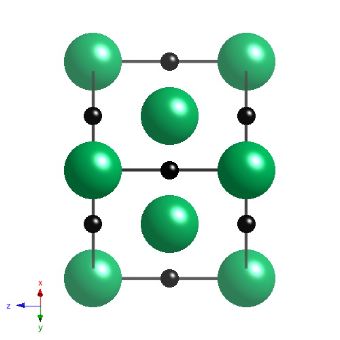
Projection of the crystal structure of NbC along a 110 direction. |
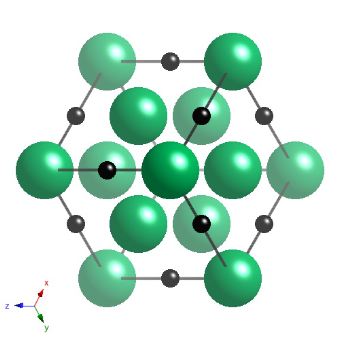
Projection of the crystal structure of NbC along a 111 type direction. |
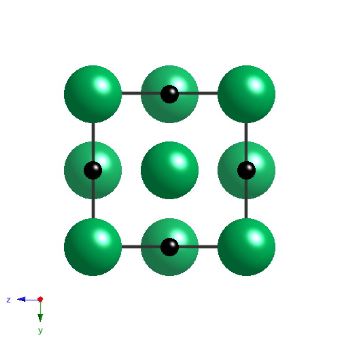
Projection of the crystal structure of NbC along a cube edge. |
|
|
Publications
- "Dissolution behaviour of NbC during slab reheating"
ISIJ International 54 (2014) 1677-1681.
Hong Yeob Lee, Kyong-Su Park, Jung Hyeung Lee, Yoon-uk Heo, Dong Woo Suh and H. K. D. H. Bhadeshia
-
"Role of Niobium in Influencing Transformation of
Microalloyed Austenite"
Metallurgical and Materials Transactions A, Vol. 26A, 1995, pp. 21-30.
C. Fossaert, G. Rees, T. Maurickx and H. K. D. H. Bhadeshia
-
"The Effect of Niobium in Solid Solution on the
Transformation Kinetics of Bainite"
Materials Science and Engineering A, Vol. A194, 1995,
pp. 179-186.
G. Rees, J. Perdrix, T. Maurickx and H. K. D. H. Bhadeshia
-
"Competitive Effects of Niobium and Niobium Carbides
on the Kinetics of the Bainite Reaction"
Proceedings of Solid-Solid Phase Transformations '94, 1994
T. Maurickx, G. Rees and H. K. D. H. Bhadeshia
-
"Modelling Precipitation of Niobium Carbide in Austenite: Multicomponent Diffusion, Capillarity and Coarsening"
Materials Science and Technology, Vol. 17, 2001, 403-408.
N. Fujita and H. K. D. H. Bhadeshia
-
"Modelling M6C Precipitation in Niobium-Alloyed Ferritic Stainless Steel"
Metallurgical and Materials Transactions A, Vol. 33A, 2002, 3339-3347.
N. Fujita, H. K. D. H. Bhadeshia and M. Kikuchi
-
"Theory for niobium in steels"
Materials Science and Technology 31 (2015) 1066-1076.
P. Yan and H. K. D. H. Bhadeshia and M. Kikuchi
Niobium and Ferro-Niobium Production
Photographs courtesy of Dr Thomas Sourmail
The main production of niobium and an alloy or iron and niobium (ferroniobium) is at CBMM, Araxá, Brazil, where there are sufficient reserves to last for 500 years at current world consumption rates. The pyrochlore ore is mined simply by digging it out from open pits - at this stage it contains about 3% of Nb2O5. This is then enriched using the floatation process. The enriched ore is then reacted with aluminium to getter the oxygen and ferroniobium. Electron-beam refining is used to produce pure noibium. Most of the niobium produced is in the form of ferroniobium, which goes towards the production of huge quantities of microalloyed steels.

The open cast mine |

Floatation process ot enhance the niobium content of the ore |

Hot ferroniobium |

Ferroniobium |

Packing tin cans of ferroniobium |

Electron beam furnace for the refinement of niobium metal, producing 210 tonnes per annum |

Electron beam refined niobium ingot |
Niobium carbide in Hardfacing Weld Deposit
The following micrographs are courtesy of Dr Mario C. Cordero-Cabrera.

Fe-1.4C-6Cr-8Nb-1Si wt% hardfacing alloy. The white particles a niobium carbides (NbC) present in a matrix of retained austenite, martensite and some M_7C_3 particles. |
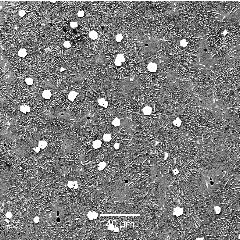
Fe-1.4C-6Cr-8Nb-1Si wt% hardfacing alloy. The white particles a niobium carbides (NbC) present in a matrix of retained austenite, martensite and some M_7C_3 particles. |

Fe-C-Nb-Si hardfacing alloy. The white particles a niobium carbides (NbC) present in a matrix of retained austenite, martensite, ferrite and some M_7C_3 particles. |












