
Consequences of a titanium fire in an aeroengine. Nickel alloy blades have been burnt away. Photograph courtesy of Dr M. Hicks, RR.

Pure titanium melts at 1670oC and has a density of 4.51 g cm-3. It should therefore be ideal for use in components which operate at elevated temperatures, especially where large strength to weight ratios are required. Titanium can catch fire and cause severe damage in circumstances where it rubs against other metals at elevated temperatures. This is what limits its application in the harsh environment of aeroengines, to regions where the temperature does not exceed 400oC.

| Consequences of a titanium fire in an aeroengine. Nickel alloy blades have been burnt away. Photograph courtesy of Dr M. Hicks, RR. |
The world production of titanium is nevertheless very small, hundreds of thousands of tonnes, which compares say with steel at 750 million tonnes per annum. 80% of all the titanium produced is used in the aerospace industries. Car suspension springs could easily be made of titanium with a great reduction in weight but titanium is not available in the large quantities needed and certainly not at the price required for automobile applications. The target price for titatnium needs to be reduced to about 30% of its current value for serious application in mass-market cars.
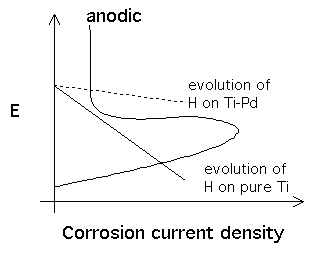
Pure titanium has excellent resistance to corrosion and is used widely in the chemical industries. There is a passive oxide film which makes it particularly resistant to corrosion in oxidising solutions. The corrosion resistance can be further improved by adding palladium (0.15 wt%), which makes hydrogen evolution easier at cathodic sites so that the anodic and cathodic reactions balance in the passive region
Pure titanium has excellent resistance to corrosion and is used widely in the chemical industries. There is a passive oxide film which makes it particularly resistant to corrosion in oxidising solutions. The corrosion resistance can be further improved by adding palladium (0.15 wt%), which makes hydrogen evolution easier at cathodic sites so that the anodic and cathodic reactions balance in the passive region. The diagram is a plot of the potential versus current density. The cathodic reaction is the evolution of hydrogen, represented by the straight lines(continuous ≡Ti, dashed ≡Ti-Pd). The corrosion current density is given by the point where the anodic and cathodic curves intersect. |
|
Most chemical plant use steel vessels which are clad with titanium. The titanium is frequently explosion bonded. Titanium condenser tubes are used in power plant and in desalination plant.
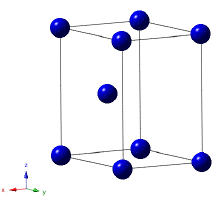
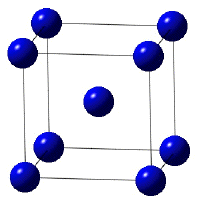
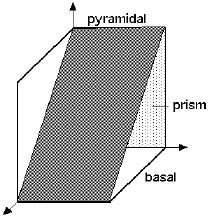
The crystal structure of titanium at ambient temperature and pressure is close-packed hexagonal (α) with a c/a ratio of 1.587. Slip is possible on the pyramidal, prismatic and basal planes in the close-packed directions. At about 890oC, the titanium undergoes an allotropic transformation to a body-centred cubic β phase which remains stable to the melting temperature.
 |
 |
 |
Crystal structure of α-titanium. |
Crystal structure of β-titanium. | The slip planes in α-titanium |
All elements which are within the range 0.85-1.15 of the atomic radius of titanium alloy substitutionally and have a significant solubility in titanium. Elements with an atomic radius less than 0.59 that of Ti occupy interstitial sites and also have substantial solubility (e.g. H, N, O, C). The ease with which solutes dissolve in titanium makes it difficult to design precipitation-hardened alloys. Boron has a similar but larger radius than C, O, N and H; it is therefore possible to induce titanium boride precipitation. Copper precipitation is also possible in appropriate alloys.
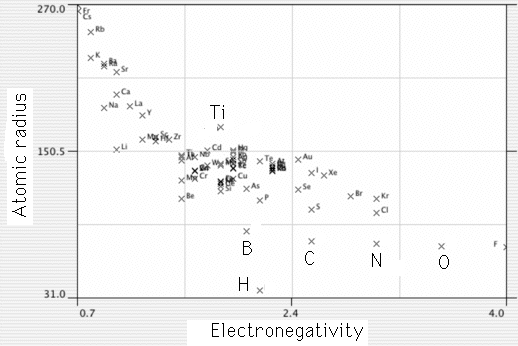 |
Plot of a measure of atomic radius versus Pauling electronegativity for elements. Notice there are many elements of similar size to titanium, and B, H, N, O and C all fall in the interstitial range of the Hume-Rothery rules. |
The alloying elements can be categorised according to their effect on the stabilities of the α and β phases. Thus, Al, O, N and Ga are all α-stabilisers. Mo, V, W and Ta are all β-stabilisers.
Cu, Mn, Fe, Ni, Co and H are also β-stabilisers but form the eutectoid. The eutectoid reaction is frequently sluggish (since substitutional atoms involved) and is suppressed.
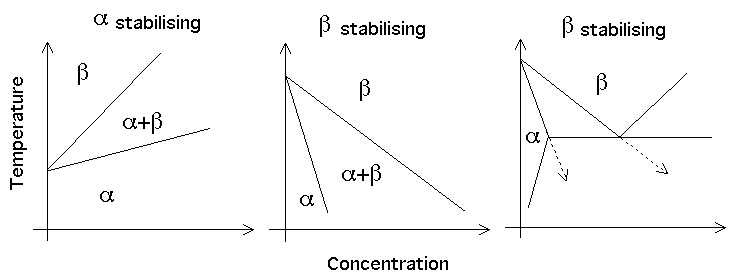 |
| Phase diagrams for titanium alloys. |
Molybdenum and vanadium have the largest influence on β stability and are common alloying elements. Tungsten is rarely added due to its high density. Cu forms TiCu2 which makes the alloys age-hardening and heat treatable; such alloys are used as sheet materials. It is typically added in concentrations less than 2.5 wt% in commercial alloys.
Zr, Sn and Si are neutral elements.
These do not fit properly and cause changes in the lattice parameters. Hydrogen is the most important interstitial. Body-centred cubic Ti has three octahedral interstices per atom whereas c.p.h. Ti has one per atom. The latter are therefore larger, so that the solubility of O, N, and C is much higher in the α phase.
Titanium is capable of absorbing up to 60 at.% of hydrogen, which can also be removed by annealing in a vacuum. Hydrogen enters the tetrahedral holes which are larger in b.c.c. than c.p.h. Thus the solubility of hydrogen is larger in β. The enthalpy of solution of hydrogen in Ti is negative (ΔH<0).
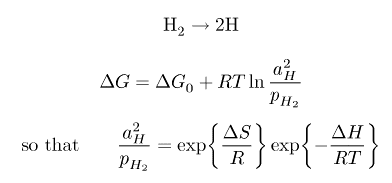
|
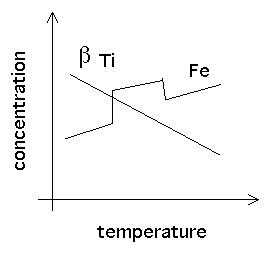
|
As shown on the graph on the right, the solubility actually decreases with temperature. This contrasts with iron which shows the opposite trend.
Because of this characteristic, titanium is a candidate material for the first wall of magnetically confined fusion reactors. The hydrogen based plasma is not detrimental since at 500oC and 1Pa pressure, the Ti does not pick up enough hydrogen for embrittlement. An additional feature is that Ti resists swelling due to neutron damage.
A large enough concentration of hydrogen induces the precipitation of hydrides. TiH1.5-2.0 has a Cubic-F lattice and its precipitation causes embrittlement due to a volume expansion of about 18%. There are regions of hydrostatic tension at crack tips where it forms preferentially, leading to large increases in the crack growth rate, some 50-fold during fatigue.
The hydride reaction can also be used to store hydrogen reversibly:
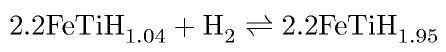
The energy to weight ratio for such a cell is about a tenth that of petrol.
One problem with this method of hydrogen storage is that hydride formation is accompanied by a considerable volume expansion, which in turn can embrittle the alloy. Amorphous alloys of titanium are better in this respect, since they do form hydrides and yet reversibly accommodate large quantitities of hydrogen by an expansion of the nearest-neighbour distance. Titanium and zirconium are metallurgically similar. The latter also forms hydrides.
Zr-Ti Laves phase Ti0.24Zr0.76(Ni0.55Mn0.3V0.065Fe0.085)2.1 has been found to reversibly accommodate nearly 1.5 wt% of hydrogen, with a battery rating of some 440 mAh g-1.
α alloys are easily welded and are relatively tough even at cryogenic temperatures temperatures. Aluminium is the main alloying element apart from Zr and Sn. The combined effect is expressed as:
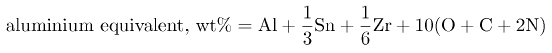
If this exceeds about 9 wt% then there may be detrimental precipitation reactions (generally Ti3X which has an ordered h.c.p. structure).
The presence of a small amount of the more ductile β-phase in nearly α alloys is advantageous for heat treatment and the ability to forge. The alloys may therefore contain some 1wt% of Mo e.g.
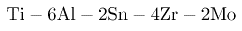
where the Zr and Sn give solid solution strengthening.
Ti-5Al-2.5Sn wt% is an α alloy which is available commercially in many forms. Because it is stable in the α condition, it cannot be hardened by heat treatment. It is therefore not particularly strong, but can easily be welded. The toughness at cryogenic temperatures increases when the oxygen, carbon and nitrogen concentrations are reduced to produce a variant designated ELI, standing for extra low interstitials. The fact that the strength increases at low temperatures, without any deterioration in toughness, makes the alloy particularly suitable for the manufacture of cryogenic storage vessels, for example to contain liquid hydrogen.
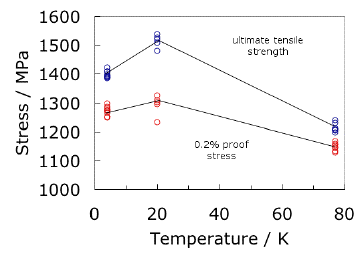 |
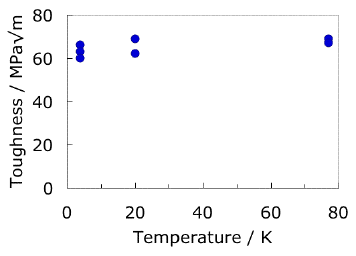 |
The results plotted above are from an ingot of Ti-5Al-2.5Sn ELI, which was forged at 1473 K (maximum), held at 1073 K for 2 hours and then air cooled. The variability in the strength data is a reflection of the position from which the test sample was extracted from the forged billet. The data are from the National Institute for Materials Science, Japan. |
|
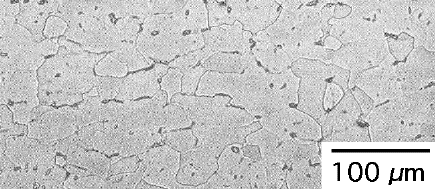 | Microstructure of Ti-5Al-2.5Sn ELI alloy in a 160 mm diameter billet (National Institute for Materials Science, Japan). It consists mostly of α with a small amount of β. |
A near-α alloy has been developed, with good elevated temperature properties (T<590oC ):
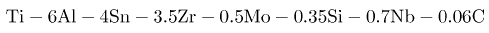
The niobium is added for oxidation resistance and the carbon to allow a greater temperature range over which the alloy is a mixture of α+β, in order to facilitate thermomechanical processing. This particular alloy is used in the manufacture of aeroengine discs and has replaced discs made from much heavier nickel base superalloys. The final microstructure of the alloy consists of equiaxed primary-α grains, Widmanstätten α plates separated by the β-phase.
Most α+β alloys have high-strength and formability, and contain 4-6 wt% of β-stabilisers which allow substantial amounts of β to be retained on quenching from the β→α+β phase fields, e.g. Ti-6Al-4V. Al reduces density, stabilises and strengthens α while vanadium provides a greater amount of the more ductile β phase for hot-working. This alloy, which accounts for about half of all the titanium that is produced, is popular because of its strength (1100 MPa), creep resistance at 300oC , fatigue resistance and castability.
 |
 |
The typical microstructure of Ti-6V-4Al wt% alloy, cooled from the α phase field to produce Widmanstätten β. The micrographs are from the DoITPoMS project, courtesy of Bill Clyne. |
One difficulty with the β phase, which has a body-centred cubic crystal structure, is that like ferritic iron, it has a ductile-brittle transition temperature. The transition temperature tends to be above room temperature, with cleavage fracture dominating at ambient temperatures.
A powder metallurgical variant of Ti-6Al-4V, containing small concentrationas of boron and carbon has been developed with an approximately 25% higher strength and modulus, but significantly lower ductility. The alloy contains stable TiB precipitates which prevent grain growth during the hot-processing operations (Adv. Mater. Proc., Oct 2005, p.9).
Titanium fires can occasionally occur in aeroengines or in titanium-based heat exchangers used in the chemical industries.
The addition of chromium in concentrations exceeding 10 wt% helps improve the burn-resistance of titanium alloys. The alloy Ti-35V-15Cr wt%, has sufficient chromium to resist burning in an aeroengine enviroment to temperatures upto about 510oC . The chromium is not found to be effective in binary Ti-Cr alloys where it does not encourage the formation of a continuous film of protective oxide.
Quenching the β phase leads to the formation of h.c.p. α' martensite. This is not particularly hard and there are increasing quantities of retained-β in the microstructure as the solute concentration increases and the MS temperature decreases.
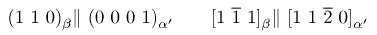
and the habit plane of the martensite is close to {3 3 4}β.
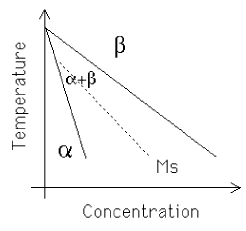 | Martensitic transformation from β. Notice that for all compositions, the transformation is suppressed below the equilibrium α+β/β phase boundary. This is because of the non-equilibrium nature of martensite. |
ω is a metastable phase which forms from β in alloys based on titanium, zirconium and hafnium. It is important because its formation generally leads to a deterioration in the mechanical properties. In Ti-Nb alloys its formation influences superconduction. The transformation to ω is diffusionless, occurs below the T0 temperature and frequently cannot be suppressed even by quenching at 11000 K s-1. Its presence causes diffuse streaking in the electron diffraction patterns of the β phase. The streaks become more intense and curved as the temperature or the solute concentration increases. There is also an increase in the electrical resistance as ω forms.
The β→ω transformation is reversible and diffusionless but is not martensitic in the classical sense since there is no invariant-plane strain shape deformation. However, it does involve the coordinated motion of atoms.
The body-centred cubic (bcc) crystal structure of β can be imagined as the stacking of {111}β planes in an ....ABCABC.... stacking sequence. Note that these planes are not close-packed in the bcc structure. The β→ω transformation occurs by the passage of a longitudinal displacement wave along <111> which causes the B and C planes to collapse into each other, leaving the A planes unaffected. The stacking sequence thus changes to ...AB'AB'AB'.... in which the B' planes have twice the density of atoms as the A planes. The ...AB'AB'AB'.... stacking is consistent with a ω a hexagonal crystal structure with a c/a of about 0.6. The atoms in the B' plane have a trigonal coordination which is similar to that in graphite and the bonding becomes partly covalent. This leads to an increase in the electrical resistivity. The longitudinal displacement waves are responsible for the streaking in the electron diffraction patterns.
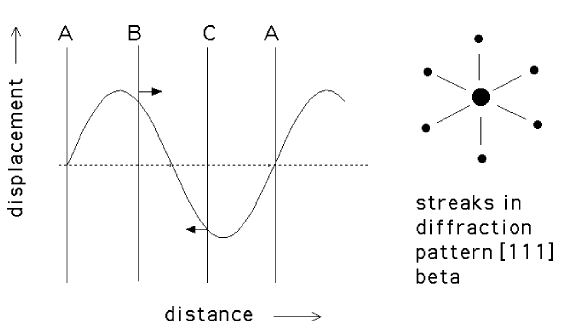 | (a) Displacement wave associated with the β to ω transformation. The A planes are unaffected since they lie at the nodes. (b) Streaks in the electron diffraction pattern during the ω transformation. |
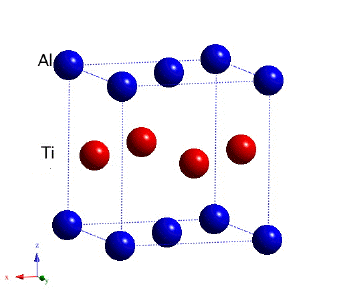
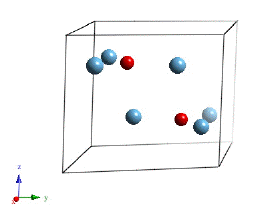
The most successful of the aluminides has a lamellar structure made up of alternating layers of an hexagonal Ti3Al α2 compound and tetragonal TiAl or γ.
 |
 |
 |
Tetragonal TiAl, γ. Movie. |
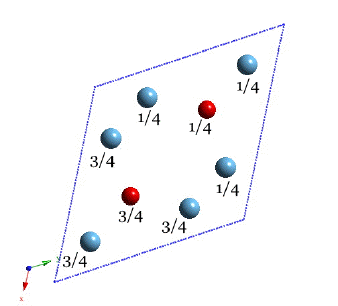
Projection of the crystal structure along the z-axis. Hexagonal Ti3Al α2. The numbers represent fractional coordinates along the z-axis. |
Hexagonal Ti3Al α2.Movie. |
The tensile ductility is about 4-6% at ambient temperature. The γ-aluminide tends to be more ductile. The density is about 4.5 g cm2 and the aluminium makes the aluminide more resistant to burning. The alloys have been studied extensively for aerospace and automotive turbochargers because of their high strength, low density and creep resistance. The γ phase forms with its most closely packed plane parallel to the basal plane of the α2:
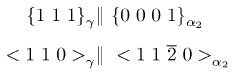
The lamellar microstructure is a direct consequence of this orientation relationship.
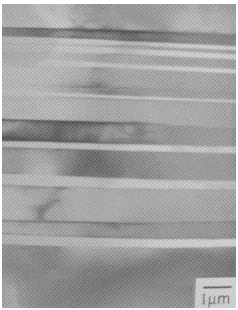 | Ti-48Al at.%: lamellar microstructure of alternating layers of α2 and γ (Kim and Maruyama, 2001) |
| Superalloys | Titanium | Bainite | Martensite | Widmanstätten ferrite |
| Cast iron | Welding | Allotriomorphic ferrite | Movies | Slides |
| Neural Networks | Creep | Mechanicallly Alloyed | Theses |
| PT Group Home | Materials Algorithms |

|

|