
Recrystallisation and grain growth involve the movement of grain boundaries. The motion will be inhibited by second phase particles. The drag on the boundary due to an array of insoluble, incoherent spherical particles is because the grain boundary area decreases when a boundary intersects the particle. Therefore, to move away from the particle requires the creation of new surface. The net drag force on a boundary of energy ![]() per unit area due to a particle of radius
per unit area due to a particle of radius ![]() is given by (Fig. 1)
is given by (Fig. 1)
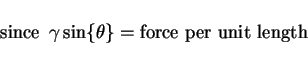 |
(1) |
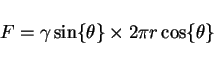 |
(2) |
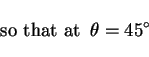 |
(3) |
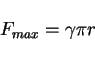 |
(4) |
|
(a)
![\includegraphics[width=5.5cm]{zener1.eps}](img8.png) (b) (b) ![\includegraphics[width=14.5cm]{zener2.eps}](img9.png)
|
Suppose now that there is a random array of particles, volume fraction
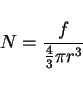 |
(5) |
Only those particles within a distance ![]() can intersect a plane. The number of particles intersected by a plane of area
can intersect a plane. The number of particles intersected by a plane of area ![]() will therefore be
will therefore be
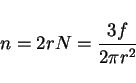 |
(6) |
The drag pressure ![]() is then often expressed as
is then often expressed as
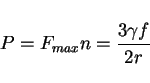 |
(7) |
This may be a significant pressure if the particles are fine. Anisotropic particles may have a larger effect if they present a greater surface area for interaction with the boundary.
A grain of radius ![]() has a volume
has a volume
![]() and surface area
and surface area ![]() . The grain boundary energy associated with this grain is
. The grain boundary energy associated with this grain is
![]() where
where ![]() is the boundary energy per unit area and we have taken into account that the grain boundary is shared between two grains. If follows that:
is the boundary energy per unit area and we have taken into account that the grain boundary is shared between two grains. If follows that:
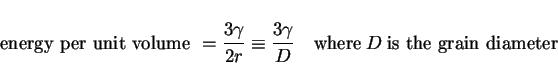 |
(8) |
It is this which drives the growth of grains with an equivalent pressure of about 0.1 MPa for typical values of
![]() and
and
![]() . This is not very large so the grains can readily be pinned by particles (Zener drag).
. This is not very large so the grains can readily be pinned by particles (Zener drag).
The micrographs below are from a steel sample of chemical composition Fe-0.06C-0.3Si-1.02Mn-0.2Cu-2.43Ni-0.2Cr-0.46Mo wt%, containing a variety of oxygen concentrations. The oxygen is in the form of fine oxide particles. The sample was metallographically polished and then heated to 1200°C to thermally etch the austenite grain boundaries. The oxide particles serve to prevent the growth of austenite grains during this heat-treatment, via Zener pinning. It is not surprising that the final austenite grain size decreases as the oxygen concentration increases.
The term ppmw stands for parts per million by weight.
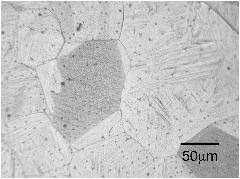 10 ppmw oxygen |
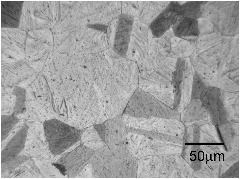 110 ppmw oxygen |
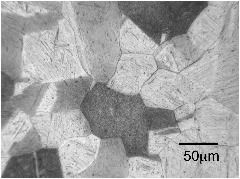 140 ppmw oxygen |
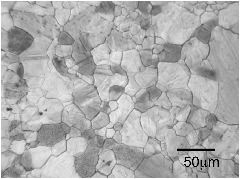 270 ppmw oxygen |
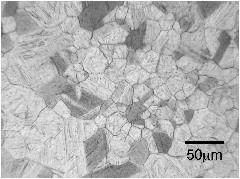 350 ppmw oxygen |
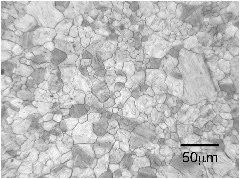 560 ppmw oxygen |