
Metallography of Aluminium-Copper Age Hardening Alloys
R. Cornell and H. K. D. H. Bhadeshia
A discussion of precipitates and precipitate-free zones can be found in a series of lectures available online.
Aluminium alloys are grouped according to the major alloying elements they contain. The 2XXX group contains copper which is added for strength achieved by precipitation hardening although the corrosion resistance tends to worsen.
Copper is added in concentrations up to about 10 wt% although alloys for structural applications usually contain smaller concentrations. Copper has a low solubility in aluminium at low temperatures. An alloy quenched from high temperatures to retain the copper in solid solution will therefore be metastable. Given an opportunity the copper will tend to precipitate. This can occur even at room temperature, so that hardness will change as a function of time, a phenomenon known as "age hardening".
The precipitation reactions in Al-Cu are quite complex. The equilibrium phase CuAl2 is difficult to nucleate so its formation is preceded by a series of metastable precipitates. Guinier and Preston first discovered many of the age hardening phenomena. The first two precipitates to form in the sequence are, therefore, known as GP zones. GP1 consists of 10 nm diameter copper-rich plates on {100}Al planes. These develop into GP2 zones which are also coherent plates 10 nm thick and 150 nm diameter. These lead to maximum hardening. Theta' precipitates then replace the GP zones as semi-coherent particles, a stage known as over-aging because the hardness begins to decrease. The equilibrium phase CuAl2 has a tetragonal crystal structure and contributes little to hardness.
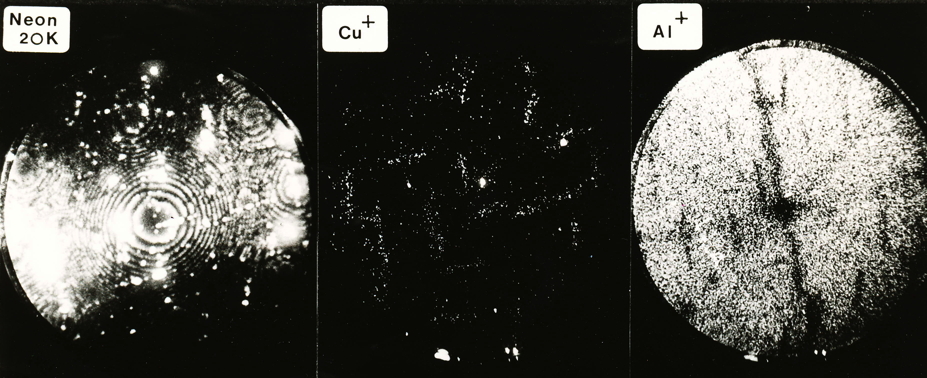
These atom-probe images were taken from Al-4Cu wt% solid solution that was aged at 130°C for 95 h to produce the GP zones illustrated by the Cu+ image. Field ion image showing the positions of all atoms, imaging atom-probe picture showing the copper atoms and corresponding aluminium atom image. Photographs courtesy of A. R. Waugh and S. Waugh.
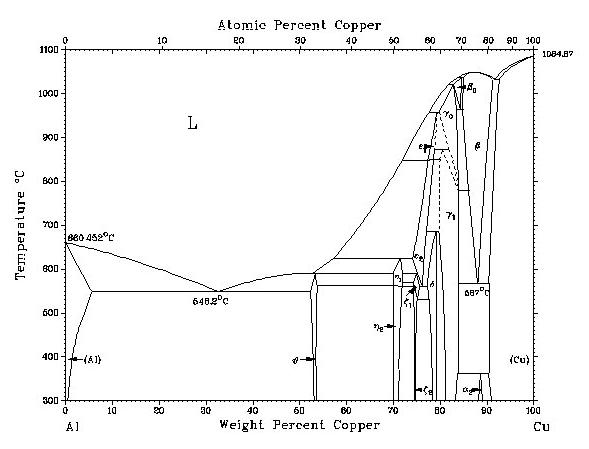 |
Aluminium-copper phase diagram, reproduced with the permission of Jud Ready of the Georgia Tech. Joint Student Chapter of ASM/TMS.
|
Only the CuAl2 are within the reach of optical microscopy. Specimen M24, which is an Al-4Cu wt% alloy, is in a grossly overaged condition to facilitate obsevations.
Al-4Cu wt% (low magnification, etched in NaOH).
 |
Shows a heterogeneous distribution of precipitates of CuAl2. There are very coarse particles at the grain boundaries and at grain boundary junctions, which are the easiest sites for heterogeneous nulceation. The grain interiors have finer precipitates. The regions near the grain boundaries are depleted in solute due to the formation of the boundary precipitates, and hence are free from precipitates.
|
Al-4Cu wt% (high magnification, etched in NaOH).
 |
The precipitate-free zones (PFZ) and grain boundary precipitates are more visible in this image. The PFZ's are obviously regions of weakness. However, they have a more serious deterimental effect. The free energy of the PFZ is different from the remaining microstructure, so that corrosion currents arise in the presence of electrolytes (water). This leads to severe and unacceptable attack of the microstructure. This can be avoided by cladding the age-hardened sample with pure aluminium, a procedure adopted for the construction of airframes.
|
Al-4Cu wt% (high magnification, etched in NaOH).
 |
The precipitate-free zones (PFZ) and grain boundary precipitates.
|
These videos contains information about the aluminium-copper alloy, placed in the context of a dramatic event during World War 1. Also, blog about airship.
Al-Al2Cu eutectic
The eutectic is at 33 wt% copper and 646.2oC.The micrographs below are courtesy of M.A. Tunes, C. Quick and P. Dumitraschkewitz from Montanuniversitaet Leoben in Austria.
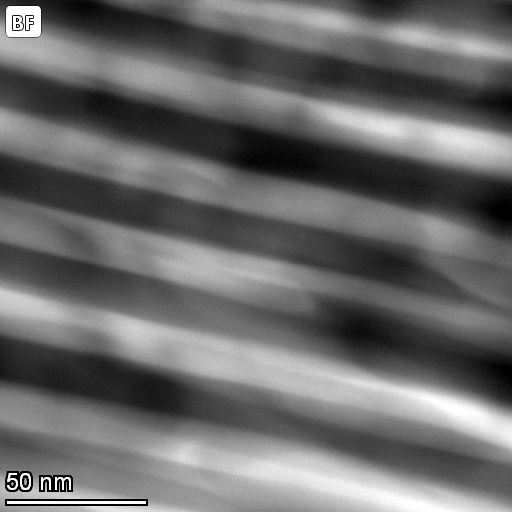 | Bright field scanning transmission electron microscope image of Al-33 wt% eutectic. |
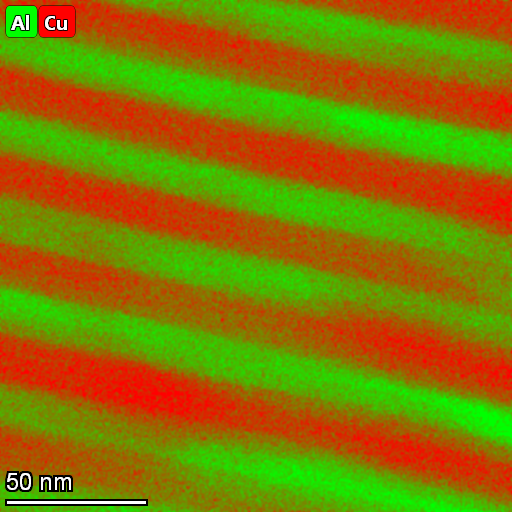 | Corresponding scanning transmission electron microscope - energy dispersive X-ray analysis image showing the distribution of elements in the two phases (aluminium solid solution and Al2Cu) eutectic. |
Anodising
Aluminium alloys are sometimes anodised This may not be appropriate for Al-Cu alloys because the anodising treatment leads to a deterioration in the fatigue properties. This is because the copper-rich regions develop pits during the early stages of treatment in anodising solutions. The pits are then initiation sites for fatigue (Materials Science and Technology, 21, 2005, pp. 1227-1235).
Links







