
The Kirkendall Effect
H. K. D. H. Bhadeshia
Vacancies
Vacancies are missing atoms in a crystal structure. The term is also used rather loosely in the context of liquids and amorphous solids. Suppose we have a perfect, pure crystal containing a mole of atoms (6.023 x 1023). There is only one distinguishable way in which the atoms can be arranged. The introduction of a single vacancy enables a very large number of alternative arrangements. The configurational entropy thus greatly increases. This favours the formation of a vacancy. Nevertheless, vacancies are defects and the associated defect energy (enthalpy of formation) opposes their formation. A compromise is reached whereby there is an equilibrium concentration of vacancies. A perfect crystal is therefore unachievable in all practical circumstances!
The equilibrium concentration is typically 10-6, i.e., one in a million of the sites is vacant, at a temperature close to melting. Pairs of vacancies, called divacancies, also exist but obviously at even lower concentrations. In platinum, the concentration of divacancies has been shown to be about 10% that of monovacancies. [Note 1] [Note 2]
Possible Mechanisms of Substitutional Atom Diffusion
Atoms can in principle migrate by direct place exhange, with the correlated motion of two adjacent atoms. But this would entail very large localised distortions in the crystal.
These distortions can be reduced by the ring diffusion mechanism, but this has the disadvantage that many atoms have to move in a correlated manner. (This mechanism is frequent in liquids and amorphous solids which have more free space).
In elements such as germanium and silicon which have a diamond cubic crystal structure with a lot of free space, the atoms may be forced into interstitial positions during diffusion.
There are many other mechanisms, but the most obvious one involves atoms jumping into vacant sites (vacancies). However, this was not accepted as a viable mechanism for a long time because the concentration of vacancies was intuitively perceived to be too small to give perceptible diffusion. The Kirkendall experiment proves the existence of vacancy diffusion in the vast majority of metallic materials. This is a remarkable example of an experiment which is ingenious, simple and conclusive.
Kirkendall Effect
The experiment applies to solids as well as to immiscible fluids. Assume that diffusion is by a vacancy mechanism so that the flow of matter is matched by an equal and opposite flow of vacancies.
Consider the diffusion couple illustrated below, between A and B, where the diffusion rates of the two species are different (|JA| > |JB|). Since the diffusion fluxes are different, there will be a net flow of matter past the inert markers, causing the couple to shift bodily with respect to the markers. This can only happen if diffusion is by a vacancy mechanism. A place exchange mechanism does not allow the fluxes to be different.
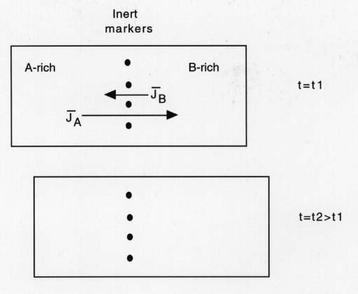 |
A diffusion couple is created by welding together A and B, with intert markers placed at the weld junction. The markers are in the form of wires extending out of the diffusion couple and attached to the laboratory bench. The whole specimen therefore translates along the bench as diffusion proceeds because the flux of A is larger than that of B.
|
Given that there is a net flow of matter there will be an equal and opposite net flow of vacancies which condense to form pores.
The light micrographs on the right illustrate a laminate of copper and nickel, both before and after heat treatment. Porosity occurs in the copper because copper diffuses faster in the nickel than vice versa. Notice that this goes against intuition because copper has a lower melting temperture than nickel. The photograph is reproduced by courtesy of Professor David Matlock of the Colorado School of Mines.
|

|
The Kirkendall Effect in Solid-State Diffusion (Ph.D. Thesis)
The full thesis can be downloaded (4 Mb) by permission of the author Aloke Paul, (aloke_paul@yahoo.co.in) Technische Universiteit Eindhoven. The contents are summarised below:
- Introduction
- Experimental Techniques
- Bifurcation and Trifurcation of the Kirkendall Plane
- Morphological Evolution
- Diffusion Studies in the β-NiAl and γ'-Ni3Al Phases
- Intermetallic Growth and Kirkendall Effect Manifestations in Cu(Ni)/Sn and Au/Sn Diffusion Couples
- Appendices
Technological Consequences: Metallic Superconductors
Multifilamentary superconducting composites are made by placing niobium in bronze (Cu-Sn) and then drawing the composite-bar into filaments. The filaments are then heat treated so that the tin reacts with the Nb to produce Nb3Sn, which is the superconductor. The production has to be like this because the Nb3Sn is too brittle do be drawn into wire form. The heat treatment for the necessary diffusion can lead to Kirkendall porosity. See J. D. Kelin, G. Warshaw, N. Duziak, S. F. Cogan and R. M. Rose, IEEE Transactions on Magnetics, volume 17 (1981) 380.
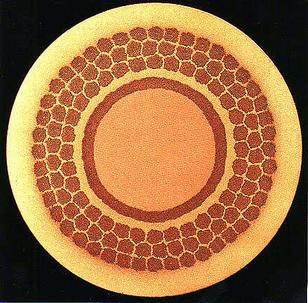
|
This picture illustrates a superconducting composite. The central core is pure copper, separated from the bronze by a diffusion barrier made of tantalum. The diffusion barrier prevents the tin in the bronze from entering the copper core. The copper core is required to control eddy currents. The outer shell of bronze contains the superconducting Nb3Sn filament bundles made by reacting the tin in the bronze with the niobium. Photograph courtesy of Professor J. E. Evetts, Department of Materials Science and Metallurgy, University of Cambridge.
|
| This high magnification view of the superconducting filaments shows Kirkendall porosity (dark dots on the perimeters of filaments). Photograph courtesy of Drs J. Robertson, J. E. Evetts and E. R. Wallach, Department of Materials Science and Metallurgy, University of Cambridge.
|
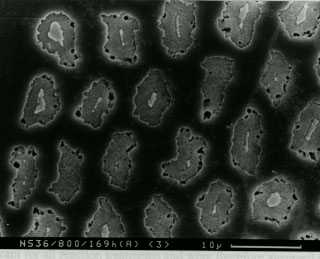
|
Technological Consequences: Shape Memory Alloys
Nickel-titanium shape memory metal is usually available in wire form, made by wire drawing. Because of the lack of ductility, it is much more difficult to fabricate the alloy in sheet form. On the other hand, both nickel and titanium are ductile in their pure states. An ingenious method of making large quantities of alloy-sheet is to make a multilayered composite of alternating plates of nickel and titanium, fabricate the composite into the final sheet form using conventional rolling technology, and then allow interdiffusion to create the nickel-titanium solid solution. The diffusion rates of nickel in titanium and titanium in nickel are different, so the process results in Kirkendall porosity. The photographs and information presented here have been provided by Professor Koichi Tsuchiya of Toyohashi University of Technology, Japan.
You can also see a movie of the shape-memory sheet in action.
Reference: D. Tomus, K. Tsuchiya, M. Inuzuka, M. Sasaki, D. Imai, T. Ohmori and M.
Umemoto: Scripta Mater. Vol. 48 (2003) 489.
Technological Consequences: Nickel-base Superalloys
Nickel base superalloys are used to make turbine blades for aeroengines. These serve in an agressive environment at temperatures in excess of 1000 °C. They are therefore coated with a nickel-aluminium alloy which has tremendous oxidation resistance, but interdiffusion at the coating/substrate interface can lead to Kirkendall porosity. See D. L. Anton and A. G. Giamei, Materials Science and Engineering, volume 76 (1985) 173. Also, J. A. Nesbitt and R. W. Heckel, Metallurgical Transactions, volume 18A (1987) 2061.
Technological Consequences: Irradiated Materials
The irradiation of a crystal (for example in a nuclear reactor) causes the creation of vacancies and interstitials. Both are mobile defects and can annhilate by migration to a sink such as a dislocation. If one of the defects migrates faster than an excess of the other is created, a condition which may lead to the condensation of the excess defect into larger defects (such as vacancy discs). Such defects are indeed found in irradiated reactor materials. See H. J. Wollenberger, "Physical Metallurgy", edited by R. W. Cahn and P. Haasen, 4th edition, Elsevier, p. 1708.
Technological Consequences: Hot-Press Forming Steels
Very strong steel (1 GPa) can be shaped by hot-press forming. In this, the steel in sheet form is heated to temperatures in the range 900-950°C for 3-10 min in order to induce it into the austenitic state. It is then formed using a press with water-cooled dies, which simultaneously shape and quench the component to martensite.
The formation of oxide scale during this process is mitigated by applying protective coatings. The micrographs below illustrate what happens to an Al-Si alloy coating containing 7-11 wt% silicon (approximately eutectic composition), as a consequence of the heating associated with hot-press forming. Interdiffusion and reaction between iron, silicon and aluminium leads to the formation Kirkendall voids.
The micrographs have kindly been provided by Dr Dong Wei Fan and Professor Bruno De Cooman. Further details are available in a paper entitled Coating Degradation in Hot Press Forming, D. W. Fan, H. S. Kim, J. K. Oh, K. G. Chin and B. C. De Cooman, ISIJ International 50 (2010) 561-568.
Field emission scanning electron micrographs showing the formation and growth of Kirkendall voids at the coating/steel interface following heat treatment as indicated in the figure captions.
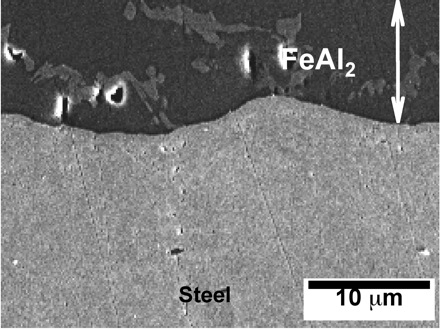
After 2 min at 930°C |

After 5 min at 930°C |
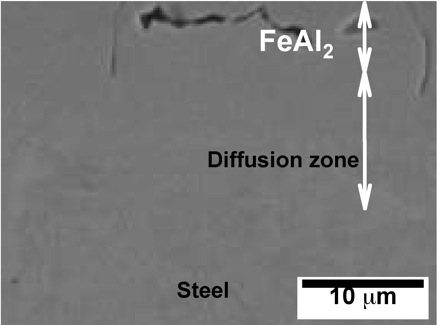
After 8 min at 930°C |
[Note 1]
Recent work [3-7] has demonstrated that the presence of interstitially dissolved hydrogen in metals such as nickel, paladium, platinum and iron can lead to the formation of many more vacancies than found under equilibrium conditions in pure metals. The resulting concentration of vacancies can be as large as 0.2. One consequence would be a reduction in density, another an increase in the diffusivity of all the atoms.
[Note 2]
The vacancy concentration can be very large (0.1), even at ambient temperature, in ordered intermetallic compounds which are not stoichiometric [8-10]. Unlike disordered alloys, there are two processes which create configurational entropy in intermetallic compounds. Atoms may occupy wrong sites which are incosistent with the order. Thus, in NiAl, the Al may occupy an Ni site and vice versa. The second is the usual change in configurational entropy when a vacancy is introduced into a perfect crystal. The large concentrations of vacancies on the nickel sublattice in off-stoichiometric NiAl_(1+x) where x is less than or equal to 1, occur because there is an increase in the number of aluminium atoms able to bond with nickel, due to the excess of aluminium [9].
Link
References
- A. D. Smigelskas and E. O. Kirkendall, Trans. AIME, volume 171, 1947, p. 130.
- I. D. Choi, D. K. Matlock and D. L. Olson, Materials Science and Engineering A, volume 124, 1990, L15-L18.
- R. B. McLellan, J. Phys. Chem. Solids, volume 49 (1988) 1213.
- R. B. McLellan, Acta Metallurgica, volume 36 (1988) 1923.
- R. B. McLellan and M. L. Wasz, J. Phys. Chem. Solids, volume 51 (1990) 523.
- Y. Fukai and N. Okuma, Japanese Journal of Applied Physics, volume 32 (1993) 1256.
- R. B. McLellan and Z. R. Xu, Scripta Materialia, volume 36 (1997) 1201-1205.
- A. J. Bradley and A. Taylor, Proc. R. Soc. A volume 159 (1937) 56.
- A. H. Cottrell, Intermetallics, volume 3 (1995) 341-345.
- X. Ren and K. Otsuka, Philosophical Magazine A, volume 80 (2000) 467-491.
- E. Kirkendall, L. Thomassen, and C. Upthegrove, Trans. AIME, 133 (1939) 186-203.
- E.O. Kirkendall, Trans. AIME, 147 (1942) 104-110.
- H. K. D. H. Bhadeshia, Strength and perfection of crystals.









