- hardenable stainless steels;
- ferritic stainless steels;
- austenitic stainless steels;
- duplex stainless steels;
- precipitation-hardened stainless steels;
- oxide dispersion-strengthened stainless steels.
- Podcast.
- Video.

Stainless steel aircraft
Steels are said to be stainless when they resist corrosion; the is achieved by dissolving sufficient chromium in the iron to produce a coherent, adherent, insulating and regenerating chromium oxide protective film on the surface. It is not surprising therefore that they are used in the harsh environments of the chemical, oil production and power generation industries, and in utility goods such as furniture, automotive trims and cutlery, where both aesthetic appearance and corrosion resistance are important design criteria.
The stainless character occurs when the concentration of chromium exceeds about 12 wt%. However, even this is not adequate to resist corrosion in acids such as HCl or H2SO4; higher chromium concentrations and the judicious use of other solutes such as molybdenum, nickel and nitrogen is then needed to ensure a robust material.
There are requirements other than corrosion which have to be considered in engineering design. For this reason, there is a huge variety of alloys available, but they can be classified into four main categories:
|
 Stainless steel aircraft |
Iron does not occur in its native state because it combines readily with oxygen and other elements. It is extracted from its ore and given the opportunity, tends to revert to a compound by reacting with the environment. Rusting is an example of this reversion process. The process can be retarded by adding chromium, which at sufficiently large concentrations forms a protective oxide film at the surface. The nature of this oxide film depends on the chromium concentration, but when the latter exceeds about 12 wt%, the a passive film of chromium oxide only about 1-2 nm thick covers the steel, which becomes stainless as long as the chromium is in solid solution in the steel.
Corrosion can nevertheless occur if the passive film breaks down, locally or uniformly:
Uniform corrosioncan occur in acidic or hot alkaline solutions. Loss by this mechanism can be estimated and allowed for in design. The corrosion rate is very slow when the metal is in the passive state.
General corrosion resistance is better at larger chromium contents, but other solutes can be detrimental. In particular, sulphur in solid solution is believed to make passivation more difficult [Schütze].
Unfortunately, sulphur alters the temperature dependence of the surface tension of liquid and in doing so increases the penetration during welding; this is very useful in during the welding of stainless steels {Llewellyn, 1991]. The fluid flow that occurs in the weld pool is such that in the absence of sulphur, shallow (wide) weld pools are obtained resulting in unacceptable joints when the concentration is less than about 0.007 wt%.
An even higher sulphur concentration may be used in free-machining stainless steels where precipitated sulphur helps break up the machining chips.
Nickel significantly improves the general corrosion resistance of stainless steels, by promoting passivation. Austenitic stainless steels therefore possess superior corrosion resistance when compared with martensitic or ferritic stainless steels (with zero or low nickel concentrations), particularly when in contact with mineral acids.
Pitting corrosionis the result of the local destruction of the passive film and subsequent corrosion of the steel below. It generally occurs in chloride, halide or bromide solutions. It can be initiated at a fault in the passive layer or a surface defect. The steel underneath the break dissolves leading to a build up of positively charged metal ions, which in turn causes negative charges ( e.g.chloride ions) to migrate near the defect. Even in a neutral solution, this can cause the pHto drop locally to 2 or 3, thereby preventing the regeneration of the passive layer.
In the passive condition, the current density is of the order of nA cm -2; in the pit, however, it may exceed 1A cm -2. The reason why the current density is so large in the pit is that the anodic region is very small in area when compared with the cathodic part (the unpitted steel). For a given corrosion current, this greatly exaggerates the corrosion rate at the pits. Similarly, the concentration of chloride ions in the vicinity of a pit can be thousands of times greater than that in the solution as a whole.
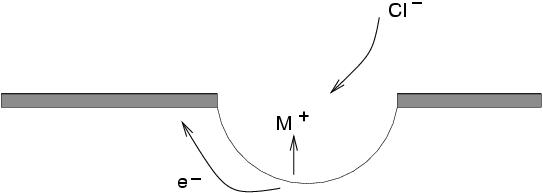 |
The above figure illustrates the process: the anodic dissolution of the steel leads to introduction of positive metal ions (M +) in solution, which causes migration of Cl -ions. In turn, metal chloride reacts with water:
M +Cl -+ H 2O -> MOH + H +Cl -
This causes the pHto decrease. The cathodic reaction, on the surface near the pit follows:
O 2+ 2H 2O -> MOH + 4OH -
While the propagation phenomenon is well understood, the mechanism of pit initiation is not. Initiation has long been associated with MnS inclusions which are difficult to avoid in the steel-making process. It appears that the inclusions are surrounded by a Cr depleted region which is believed to cause the initiation [Ryan et al., 2002].
Increasing the Cr content, or adding Mo or N enhances the pitting resistance. The potency of a solute in this respect is expressed empirically in terms of their weight percentages as a pitting index:
pitting index for duplex stainless steels =Cr+3.3Mo+16N
pitting index for austenitic stainless steels =Cr+3.3Mo+30N
One obvious environment where pitting corrosion is of concern is marine applications. Type 316 austenitic stainless steel (18Cr-12Ni and 2-3 wt% Mo) is often the material of choice in this case. When the marine requirements are particularly severe, as with offshore platforms, alloys with molybdenum concentrations up to 5 wt% are used. for example, call for heavily alloyed steels with up to 6 wt% Mo. Street furniture is another case where pitting resistance might be relevant, particularly in colder regions where salt de-icing is common.
 Stainless steel in boat |
 The ice-breaker in the background has a stainless steel hull. |
 The exposed railings are all stainless steels. |
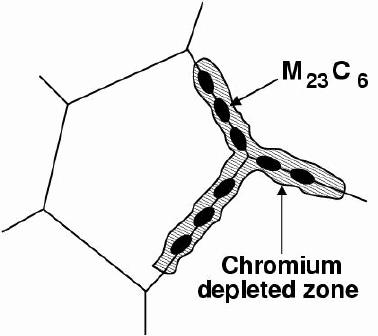
Sensitisationis one of the corrosion mechanisms which causes widespread problems in austenitic stainless steels, particularly in welded assemblies. This problem can be so severe as to cause grain decohesion, as shown Fig. 2.
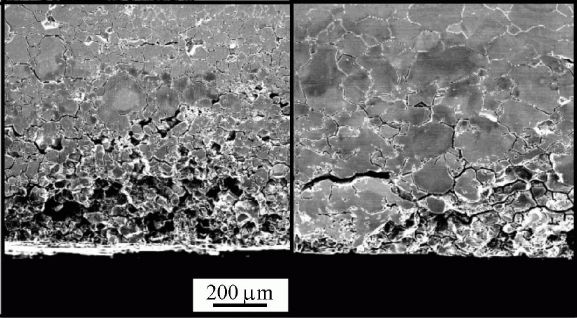 |
In normal conditions, austenitic stainless steels are given a high-temperature heat-treatment, often called a solution-treatment, which gives a fully austenitic solid solution. However, at temperatures below about 800°C, there is a tendency to precipitate chromium-rich carbides as the alloy enters the carbide plus austenite phase field.
The main carbide phase is M 23C 6, where the 'M' stands for a mixture of metal atoms including iron, molybdenum, chromium and manganese, depending on the steel composition and heat-treatment. These carbides require long-range diffusion in order to precipitate and hence can be avoided by rapid cooling from the solution-treatment temperature.
The precipitation of M 23C 6and M 7C 3occurs primarily at the austenite grain surfaces which are heterogeneous nucleation sites; it can occur in a matter of minutes at temperatures around 750°C. The chemical composition in the vicinity of the grain boundaries can be altered by the precipitationof the chromium-rich particles. The resulting chromium-depleted zone at the grain boundaries makes them susceptible to intergranular anodic-attack even under stress--free conditions. Once again, the anodic regions present a much smaller area (grain boundaries) compared with the rest of the exposed surface which is cathodic; the localised rate of corrosion at the boundaries is therefore greatly exaggerated. This is the essence of sensitisation.
Sensitisation in the context of welded samples leads to the phenomenon of weld decay. Regions are created in the heat-affected zones of the welds which precipitate carbides, become sensitised and fail by localised corrosion, almost as if the weld is unzipped in the sensitised region.
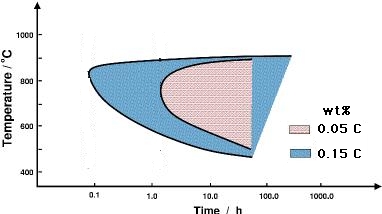
Figure 3 shows that the steel is safe from sensitisation at low times because precipitation has not yet occurred with a vengence. Prolonged heat treatment makes the steel safe by permitting diffusion to eliminate chromium concentration gradients in the austenite.
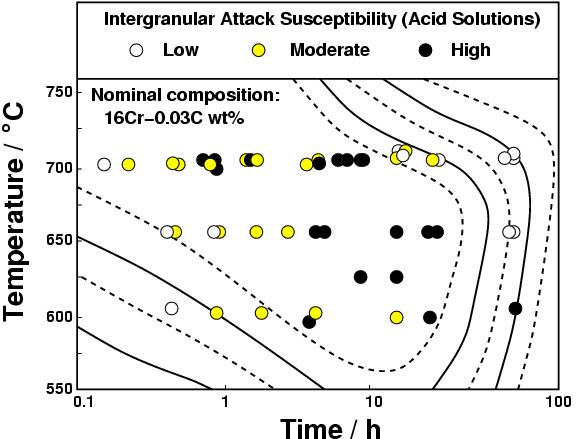 |
A variety of solutions exist to avoid sensitisation:
The first one is to reduce the carbon content of the steel, making it more difficult to precipitate carbides. Stainless steels with an 'L' associated with their numerical designation ( e.g.,304L and 316L) have been manufactured with carbon cocentrations less than about 0.03 wt%, which compares against the normal grades which typically have some 0.08 wt% of carbon. Figure 4 shows how carbon accelerates sensitisation.
 |
An alternative is use solutes (such as Nb, Ti, V or Ta) which have a greater affinity for carbon than chromium. These are called stabilisedstainless steels, for example, types 321 (Ti stabilised) and 347 (Nb stabilised) austenitic stainless steels. Titanium cannot in general be used to make alloys deposited by arc welding because it readily oxidises; type 347 is used instead as a filler metal. In welding applications, grade 321 is not used as a filler metal because titanium does not transfer well across a high temperature arc. Niobium stabilised 347 is used instead as a filler metal.
Stabilisation involves more than just an addition of Nb or Ti. A heat-treatment must be performed to stimulate the formation of TiC or NbC, for example by hoding at 900°C for one hour. This is because during lower temperature heat treatments, M 23C 6may form faster than TiC or NbC.
In some cases, a solution-treatment can be given after fabrication to dissolve carbides which may have formed on grain boundaries.
A variety of other factors impact on the problem, such as the austenite grain size and the crystallographic character of the grain boundaries. Sensitisation can be avoided by grain boundary engineering (Shimada et al.,2002), by creating a crystallographic textures which favours low-energy boundaries which are less effective as heterogeneous nucleation sites. A reduction in the austenite grain size can also help by increasing the number density of any carbides and hence reducing the extent of associated Cr diffusion fields.
As explained earlier, sensitisation is caused by the formation of chromium carbides on grain boundaries. The precipitates absorb chromium from the adjacent austenite causing a localised breakdown in passivity.
 |
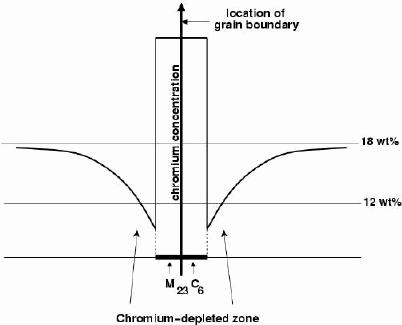 |
This short description of the problem hides most of its interesting complexity. The minimum chromium concentration reached in the austenite ajacent to the carbide is in principle determined by the appropriate part of the phase diagram, which predicts that the chromium content of the austenite in equilibrium with M 23C 6(c γM23C6) is slightly lower than the bulk composition. However, the minimum concentration reached in the austenite is smaller than indicated by the phase diagram because of multicomponent diffusion effects, the dynamics of the solute fluxes towards the precipitates.
Environmentally assisted cracking(EAC) is a generic term used to describe the consequences of a three--fold interaction between stress, environment and microstructure, an interaction which leads to unexpected failure with no ductility, usually involving a period of slow crack growth prior to final failure.
Failure occurs at applied stresses well below the macroscopic yield strength. The stress can be due to factors other than the intended design stress, for example, residual stress induced during fabrication.
An aqueous environment is required in the form of immersion or via a thin film on the surface when the component is exposed to humid atmospheres. Dissolved oxygen and anionic species such as chlorides and fluorides accelerate EAC.
Some forms of this kind of cracking can be particularly dangerous because it may take thousands of hours for a crack to nucleate, but considerably less for it to propagate. Dramatic examples of catastrophic failure include the collapse of swimming pool ceilings becuase of the stress corrosion cracking o Type 304 or 316 austenitic stainless steels. For this reason, it has been suggested that 6 wt% Mo austenitic stainless steels should be used in these environments.
Recent work has shown that it is also possible to sensitise titanium--containing stainless steels by the grain boundary precipitation of Ti(C,N) at 1100°C, by a microgalvanic mechanism (Joe and Kim, 1999).
A sensitised steel becomes more sensitive to EAC when impurities such as sulphur and phosphorus segregate to the austenite grain boundaries (McIntyre et al., 1996).
The designation stainless steelimplies little more than a 12% Cr content. Most of the stainless steels are based on the Fe-Cr-C and Fe-Cr-Ni-C systems, but other alloying elements are also important.
Iron and its alloys can exist in several crystallographic forms , of which the most common are the body-centred cubic (b.c.c.) and face-centred cubic (f.c.c.). In pure iron, the f.c.c. structure persists between 910 and 1400°C, the b.c.c. structure below and above this interval (up to the melting temperature of 1539°C).
The importance of this phase-transformation in the metallurgy of steels cannot be overestimated. This transformation allows a wide range of microstructures to be achieved by controlled heat-treatment. Mechanical properties are essentially related to microstructure, and can therefore be obtained in an extraordinarily large range of strength, toughness, etc.. Stainless steels are routinely produced with strengths from 100 MPa to more than 1 GPa.
Knowledge of the relative stabilities of the b.c.c. and f.c.c. structures of iron alloys is therefore of prime concern. The history of stainless steels started with a martensitic steel (12Cr-0.1C wt%) in Sheffield, UK and the austenitic 18Cr-8Ni wt% in Germany. For this reason, and also because they are most important alloying elements in stainless steels, Cr and Ni form the reference relative to which the influence of other solutes is classified: have long been used as reference to quantify the influence of alloying elements on the b.c.c.<->f.c.c. phase transition: those elements which like Cr promote ferrite are called ferrite stabilisersand those which like Ni promote austenite are called austenite stabilisers. The equations below give a rough guide of the potency of individual elements to act as ferrite or austenite stabilisers when compared with the corresponding effects of Cr and Ni respectively (concentrations in wt%):
Cr equivalent = (Cr) + 2(Si) + 1.5(Mo) + 5(V) + 5.5(Al) + 1.75(Nb) + 1.5(Ti) + 0.75(W)
Ni equivalent = (Ni) + (Co) + 0.5(Mn) + 0.3(Cu) + 25(N) + 30(C)
Figure 4 shows that an excessive amount of chromium can eliminate austenite at all temperatures, making it impossible to achive a γ to α transition. This is the domain of the ferritic stainless steels discussed below.
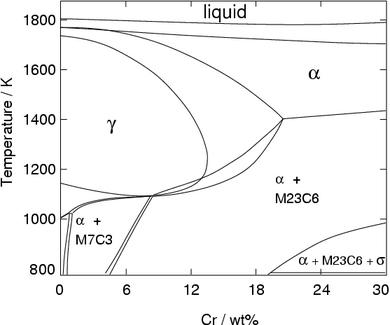 |
Without carbon, the limit beyond which austenite no longer forms is about 13.5 wt% chromium. However, additions of carbon help stabilise the austenite and therefore increase this limit (Fig. 6).
Chromium and nickel equivalents are also used in the welding industry to plot the microstructures obtained when a weld solidifies and cools to ambient temperature (Fig. 7). Although these diagrams are popular, it should be understood that they are not phase diagrams but rather represent the microstructures obtained under specific cooling conditions.
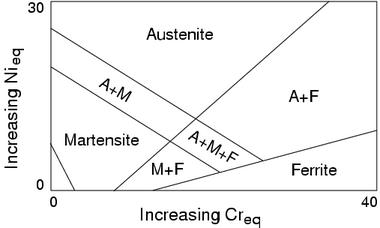 |
These include carbides, nitrides or intermetallic compounds. Since most stainless steels serve at ambient temperature, the intermetallic compounts are of little relevance as they are extremely slow to precipitate because even though they may be thermodynamically stable phases, they are difficult to nucleate.
It is evident from Figure 6 (Fe-Cr-C phase diagram) that typical martensitic steels should exhibit ferrite and M 23C 6in equilibrium at for example, 600°C. In practice, this carbide is only found after relatively long ageing. because it is preceded by Intermediate phases in the sequence cementite, M 2X and M 7C 3, leading finally to M 23C 6.
These precipitation sequences become more complex in heavily alloyed ferritic or austenitic stainless steels, such as those destined for the power generation industry. Considerable effort is being devoted to understanding and estimating the precipitation sequences in such alloys because the are intended to serve safely for 30 or more years, i.e.,for time periods far in excess of what can be reasonably achieved in the alloy development exercise (Robson and Bhadeshia, 1997; Fujita and Bhadeshia, 2002; Sourmail, 2001; Sourmail and Bhadeshia, 2003).
Most stainless steels have a high hardenability, meaning that the reconstructive transformation of austenite to (ferrite + carbides) is unlikely to happen unless the steel is cooled particularly slowly.
The most important features of these martensitic alloys are therefore the martensite start (M S) and finish temperatures (M F). For martensitic steels, the range [M S-M F] should be above the room temperature to ensure fully martensitic structure. On the contrary, the [M S-M F] range of austenitic stainless steels is often well below 0°C, which is why they can be used in cryogenic applications; austenite does not have the classical ductile-brittle transition associated with body-centred cubic iron (martensite, ferrite). Cold deformation can induce martensitic transformation to α and ε martensite, the extent depending on the strain and on the chemical composition. Heavily alloyed austenitic steels with up to 20Cr and 25Ni wt% are fully stable.
On the basis of their main microstructural features, there exist the following key categories of stainless steels:
The composition is such that the austenite in these steels is able to transform into martensite. This allows a degree of control on the mechanical properties by exploiting the phase change. Typical heat-treatments consist of austenitisation at a temperature high enough to dissolve carbides followed by quenching to obtain martensite. Given the high hardenability inherent in such alloys, the quench rate required to achieve martensite is not high; oil and water quenching are used only when dealing with thick sections.
Typical compositions cover 12 to 18 Cr and 0.1 to 1.2 C wt%. As with other martensitic steels, a balance must be sought between hardness and toughness. An untempered martensitic structure typically is strong but lacks toughness and ductility to an extent which depends on the carbon concentration. As a conseqnece, the martensite is in many cases tempered between 600 and 750°C to optimise the mechanical properties.
In applications such as cutlery, surgical instruments etc., high strength is desirable and toughness/ductility are of little concern. A lower temperature tempering is then used to retain most of the strength. Type 420 steel (0.15-0.4C, 1.0Mn, 1.0Si, 0.04P, 0.03S, 12-14Cr wt%) is a typical composition for such applications. Its proof strength in the quenched and tempered condition can be in excess of 1.2 GPa. Type 440C tempered at 300°C has a proof strength of about 2 GPa.
Table 1 shows the compositions and typical uses of AISI standard martensitic grades:
| Grade | C | Mn | Si | Cr | Ni | Mo | P | S | Comments/Applications |
|---|---|---|---|---|---|---|---|---|---|
| 410 | 0.15 | 1.0 | 0.5 | 11.5-13.0 | - | - | 0.04 | 0.03 | The basic composition. Used for cutlery, steam and gas turbine blades and buckets, bushings... |
| 416 | 0.15 | 1.25 | 1.0 | 12.0-14.0 | - | 0.60 | 0.04 | 0.15 | Addition of sulphur for machinability, used for screws, gears etc.416 Se replaces suplhur by selenium. |
| 420 | 0.15-0.40 | 1.0 | 1.0 | 12.0-14.0 | - | - | 0.04 | 0.03 | Dental and surgical instruments, cutlery.... |
| 431 | 0.20 | 1.0 | 1.0 | 15.0-17.0 | - | 1.25-2.0 | 0.04 | 0.03 | Enhanced corrosion resistance, high strength. |
| 440A | 0.60-0.75 | 1.0 | 1.0 | 16.0-18.0 | - | 0.75 | 0.04 | 0.03 | Ball bearings and races, gauge blocks, molds and dies, cutlery. |
| 440B | 0.75-0.95 | 1.0 | 1.0 | 16.0-18.0 | - | 0.75 | 0.04 | 0.03 | As 440A, higher hardness |
| 440C | 0.95-1.20 | 1.0 | 1.0 | 16.0-18.0 | - | 0.75 | 0.04 | 0.03 | As 440B, higher hardness |
In addition to the standard grades, a large number of alloyed martensitic stainless steels have been developed for moderately high temperature applications. Most common additions include Mo, V and Nb. These lead to a complex precipitation sequence. A small amount (up to 2 wt%) of Ni is added to improve the toughness.
The 12Cr-Mo-V-Nb steels are used in the power generation industry, for steam turbine blades operating at temperatures around 600°C. Current research focuses on achieving service temperatures of 630-650°C under a stress of 30 MPa.
Ferritic stainless steels: typically contain more chromium and/or less carbon than the martensitic grades. Both changes act to stabilise ferrite, so much so that it is the stable phase at all temperatures. Therefore, unlike the martensitic grades, ferritic stainless steels cannot be hardened by heat-treatment. They exhibit lower strength but higher ductility/toughness. Typical applications may include appliances, automotive and architectural trim ( i.e.,decorative purposes), as the cheapest stainless steels are found in this family (type 409).
| Grade | C | Mn | Si | Cr | Mo | P | S | Comments/Applications |
|---|---|---|---|---|---|---|---|---|
| 405 | 0.08 | 1.0 | 1.0 | 11.5-14.5 | - | 0.04 | 0.03 | 0.1-0.3 Al |
| 409 | 0.08 | 1.0 | 1.0 | 10.5-11.75 | - | 0.045 | 0.045 | (6xC) Ti min |
| 429 | 0.12 | 1.0 | 1.0 | 14.0-16.0 | - | 0.04 | 0.03 | |
| 430 | 0.12 | 1.0 | 1.0 | 16.0-18.0 | - | 0.04 | 0.03 | |
| 446 | 0.20 | 1.5 | 1.0 | 23.0-27.0 | - | 0.04 | 0.03 | 0.25 N |
Iron-chromium body-centred cubic solutions are such that there is a tendency under appropriate conditions for like atoms to cluster; at temperatures below a critical value, the solution tends to undergo spinodal decomposition into chromium-rich and iron-rich regions. High chromium ferritic stainless steels such as type 446 thus become susceptible to the so-called '475°C embrittlement', which is caused by this clustering process. At and below 475°C, in steels containing more than 25 wt% of chromium, the spinodal typically exhibits a wavelength of about 10 nm. There is a continuous increase of hardness as the composition wave develops, for example, the hardness of an Fe-28Cr wt% steel can increase by more than 300 HV over an exposure 10,000 h at 450°C (Ishikawa et al.,1995). This results in a severe drop of impact toughness and ductility.
The addition of nicikel appears to accelerate the spinodal and raises the maximum temperature at which it is observed. When post-weld heat-treatment is not possible, the welding of ferritic stainless steels is usually done with a metal filler containing Ni, and there is therefore the possibility of weld embrittlement on prolonged exposure at elevated temperatures.
These steels are often in a metastable austenitic state at room temperature or below. Most grades have a martensite-start temperature well below 0°C. However, plastic deformation can induce martensite at temperatures higher than M S(the sample then is attracted by a magnet since the α-martensite is ferromagnetic whereas austenite in such alloys is not). The M Dtemperature is that at which martensite cannot be induced no matter how much the austenite is deformed.
The presence of nickel improves considerably the corrosion resistance when compared to the martensitic and ferritic grades.
Type 304 is the basic 18Cr8Ni (18/8) austenitic stainless steel, so widely used that it accounts for about 50% of all stainless steel production. Other standard grades have different preferred applications; for example, type 316 which contains up to 3 wt% Mo, offers an improved general and pitting corrosion resistance, making it the material of choice marine applications and coastal environments. In severe conditions however, even type 316 cannot cope and molybdenum-enriched alloys such as 254SMO are used.
| AISI grade | C max. | Si max. | Mn max. | Cr | Ni | Mo | Ti | Nb | Al | V |
|---|---|---|---|---|---|---|---|---|---|---|
| 301 | 0.15 | 1.00 | 2.00 | 16-18 | 6-8 | |||||
| 302 | 0.15 | 1.00 | 2.00 | 17-19 | 8-10 | |||||
| 304 | 0.08 | 1.00 | 2.00 | 17.5-20 | 8-10.5 | |||||
| 310 | 0.25 | 1.50 | 2.00 | 24-26 | 19-22 | |||||
| 316 | 0.08 | 1.00 | 2.00 | 16-18 | 10-14 | 2.0-3.0 | ||||
| 321 | 0.08 | 1.00 | 2.00 | 17-19 | 9-12 | 5 x %C min. | ||||
| 347 | 0.08 | 1.00 | 2.00 | 17-19 | 9-13 | 10 x %C min. | ||||
| E 1250 | 0.1 | 0.5 | 6.0 | 15.0 | 10.0 | 0.25 | ||||
| 20/25-Nb | 0.05 | 1.0 | 1.0 | 20.0 | 25.0 | 0.7 | ||||
| A 286 | 0.05 | 1.0 | 1.0 | 15.0 | 26.0 | 1.2 | ~1.9 | ~0.18 | ~0.25 | |
| 254SMO | 0.02 | 0.8 | 1.0 | 18.5-20.5 | 17.5-18.5 | 6-6.5 | ~1.9 | ~0.18 | ~0.25 | |
| AL-6XN | 0.03 | 1.0 | 2.0 | 20-22 | 23.5-25.5 | 6-7 |
 Grain structure of an austenitic stainless steel NF709 (25Cr20Ni) . Many of the grains contain annealing twins. NF709 is a creep-resistant austenitic stainless steel used in the construction of highly sophisticated power generation units. |
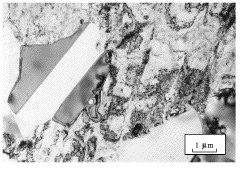 Type 302 austenitic stainless steel, cold-rolled and then annealed at 704°C for one hour. Partially recrystallised microstructure. Recrystallised grains are clean whereas the deformed regions show a large concentration of defects. There are annealing twins in the recrystallised region (Hopkin). |
Specialist austenitic stainless steels are made with up to 0.4 wt% nitrogen when prepared at ambient pressure, and up to 1 wt% nitrogen using high-pressure melting techniques (Simmons, 1996). The prime reason for adding nitrogen is that it is a very effective solid-solution strengthener. Not only do the misfitting nitrogen atoms interfere statically with moving dislocations, but there is also a drag due to nitrogen atoms being carried along with the dislocations as they move through the lattice (Rawers and Grujicic, 1966). The strength of such alloys makes them suitable for niche applications such as power generator retaining rings, high-strength bolts and superconducting magnet housings.
The solubility of nitrogen in austenite is reduced by nickel but increased by chromium and manganese. Excessive nitrogen concentrations can lead to precipitation, particularly of chromium nitrides.
Nitrogen also increases the resistance to localised corrosion (pitting and crevice) in acid-chloride solutions (see pitting index equation above).
 Stainless steel, thermally insulated, cups and saucers |
 Austenitic stainless steel |
 (a) Duplex stainless steel, IC378, hot rolled in the direction indicated. The darker etching phase is ferrite and the remainder is austenite |
 (b) Duplex stainless steel IC381 (dark phase is ferrite). |
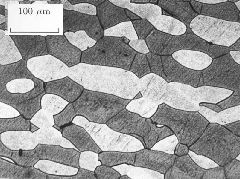 (c) Duplex stainless steel IC381 (dark phase is ferrite). |
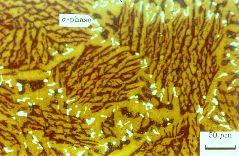 (d) Superduplex stainless steel A219 after heat treatment at 1150°C for 2.5 h. The austenite is yellow and ferrite is dark brown, with the sigma phase white. |
The archetypal duplex alloys contain 22-23Cr, 4.5-6.5Ni and 3-3.5Mo wt%, representing some 80% of all duplex stainless steel use. Detailed compositions are given in Table 4. A significant application is in the production of tubing in corrosive oil and gas wells; such tubes have been installed particularly in the North Sea industries.
| Designation | Cr | Ni | C | Mn | Si | P | S | Other | UTS / MPa | Elongation / % |
|---|---|---|---|---|---|---|---|---|---|---|
| Type 329 | 28.0 | 6.0 | 0.10 | 2.0 | 1.0 | 0.04 | 0.03 | 1.5 Mo | 724 | 25 |
| Type 326 | 26.0 | 6.5 | 0.05 | 1.0 | 0.6 | 0.01 | 0.01 | 0.25 Ti | 689 | 35 |
| 2RE60 | 18.5 | 4.5 | 0.02 | 1.5 | 1.6 | 0.01 | 0.01 | 2.5 Mo | 717 | 48 |
| IC378 | 21.8 | 5.5 | 0.03 | 1.38 | 0.40 | 0.03 | 0.01 | 3.0 Mo 0.18 Cu 0.07 V 0.14 N | ||
| IC381 | 22.1 | 5.8 | 0.02 | 1.92 | 0.48 | 0.03 | 0.01 | 3.2 Mo 0.07 Cu 0.13 V 0.14 N | ||
| A219 | 25.6 | 9.4 | 0.03 | 0.70 | 0.60 | 0.02 | 0.01 | 4.1 Mo 0.27 N |
In general, the toughness of stainless steels increases in the order ferritic, duplex and austenitic stainless steels. Duplex stainless steels, because of their high Cr concentration, are prone to the 475°C embrittlement described earlier so their application is frequently confined to temperatures below about 300°C.
The superduplex stainless steelshave a higher chromium and molybdenum concentration to enhance pitting corrosion resistance; these ferrite stabilising elements are balanced using a higher nickel and nitrogen concentrations (austenite stabilisers) in order to maintain about equal amounts of ferrite and austenite (Table 4, Fig. 8d). One definition is that a superduplex stainless steel must have a pitting index which is greater than 40.
 |
 Published 2025 |
 Published 2024 preview, video |
 Free download |
 Free download |
 Rails, 2024 |
 Translation, 2020 |
 Published 2021 |
 Published 2026 video |
 In the subway, Taipei |
 In the subway, Taipei |
This is an example of a glass and stainless steel bridge, taken from the Science Museum in London. The toughned-glass slats are structurally supported by stainless steel ropes. The ropes are usually made from pearlitic steel, but here cost is not a major issue since this is a museum exhibit.
Glass bridge supported by stainless steel ropes |
At the Science Museum, London |
 |
 |
 |
 |
 |
 |
 |
 |
 Stainless steel island, Mur, Graz |
 Stainless steel plant frame |
 Stainless steel barriers in Shanghai |
 Large stainless steel rings |
 |
 |
 |
 |
 |
 |
 409L Ferritic stainless steel: low cost, low thermal expansion and high corrosion resistance |
 |
Movie inside an elevator faced with stainless steel, in Mumbai, India
Laser welding of medical devices |
Solidification of stainless steel |
Niobium in stainless steel |
Stainless steel jewellery |
The creation of this document was supported in part by the Higher Education Funding Council for England, via the U.K. Centre for Materials Education.
| Ludlum | Join | World | Chemistry | Ulbrich |
| Carpenter | eFunda | Rust | Hendrix | Sandvik |
| Bar | Fabrication | Magnet |
| PT Group Home | Materials Algorithms |